Top 4 eConsent Questions in Clinical Research: Forms & More
Por um escritor misterioso
Descrição
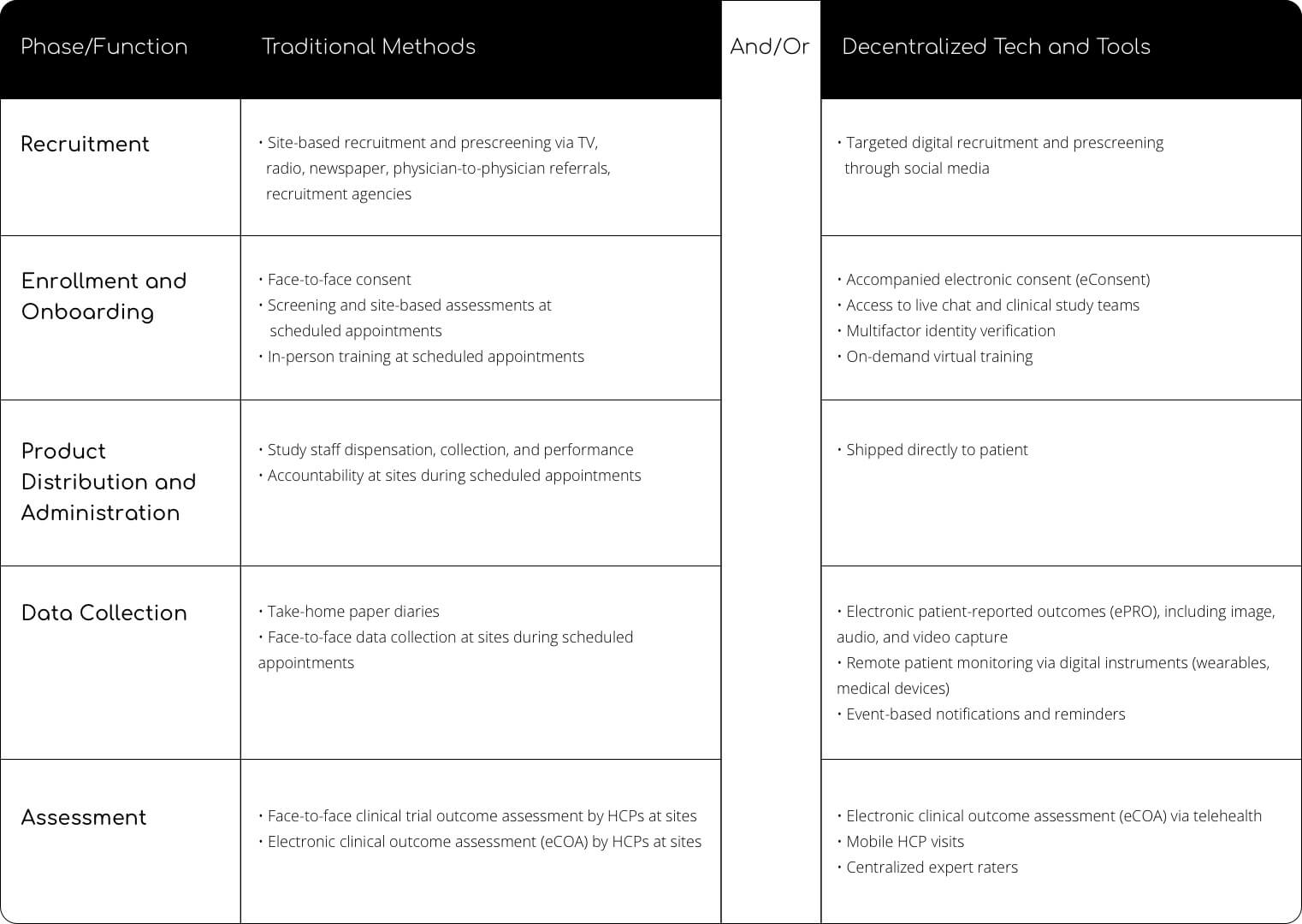
Hybrid Clinical Trials
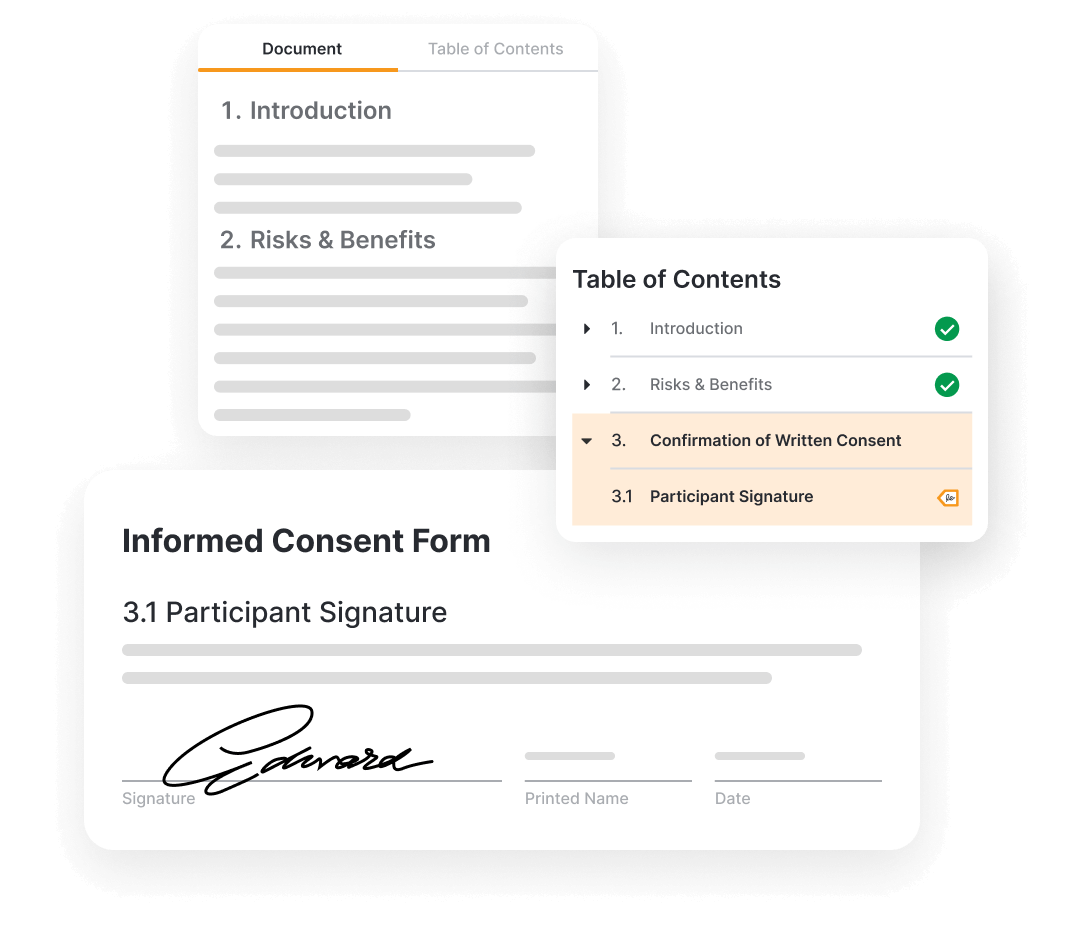
Veeva eConsent, End-to-End eConsent Platform

Patient Centric Clinical Trials and Use of eConsent, ePRO & eCOA

e-Consent in UK academic-led clinical trials: current practice
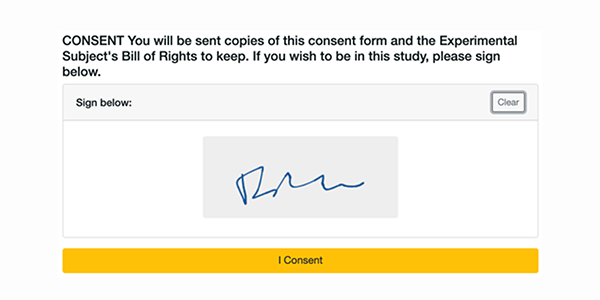
Using e-Consent Forms in Your Clinical Research
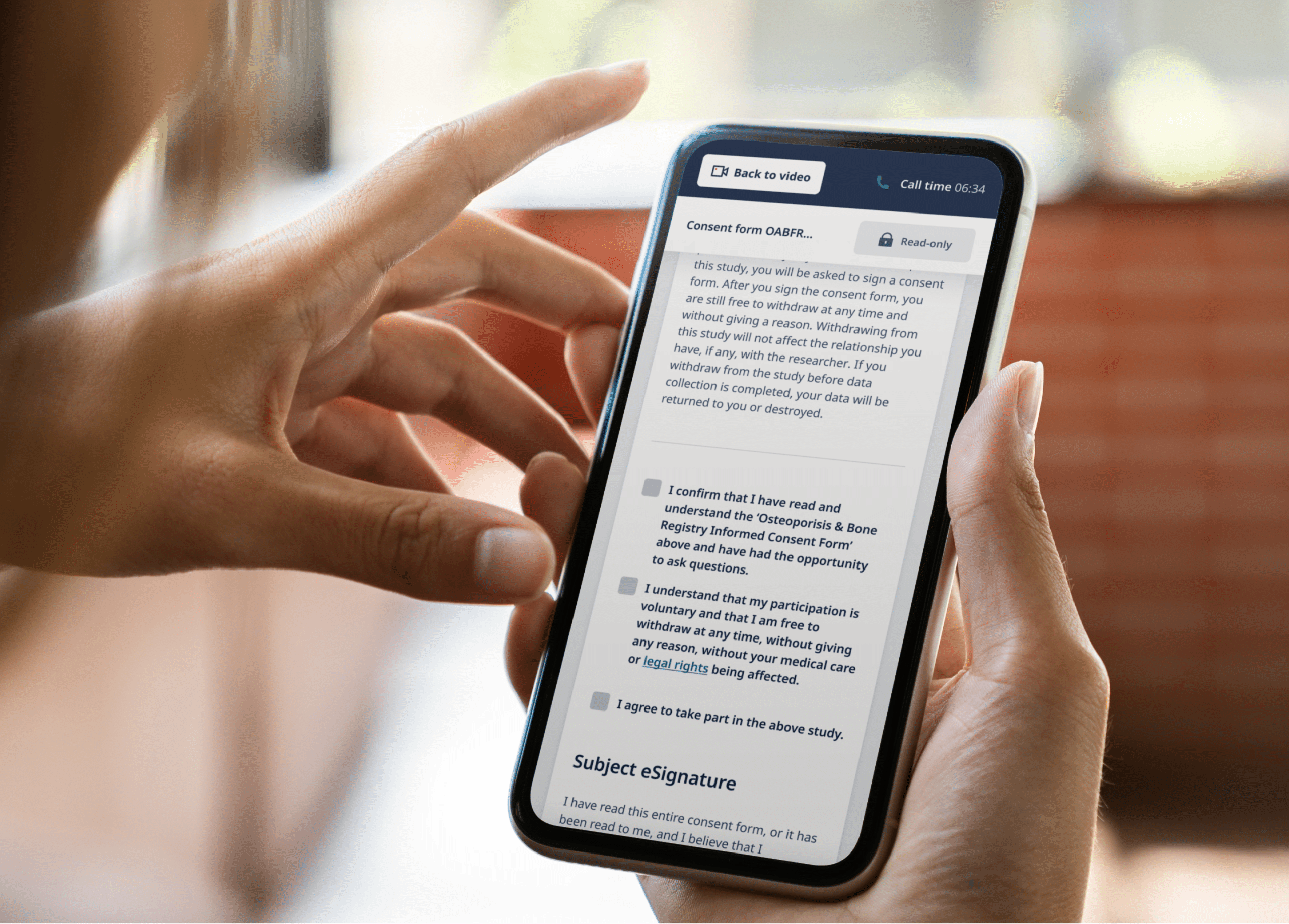
The True Value of eConsent

E-Consent—a guide to maintain recruitment in clinical trials

Awareness and Collaboration Across Stakeholder Groups Important

How To Measure What Matters in Clinical Trials
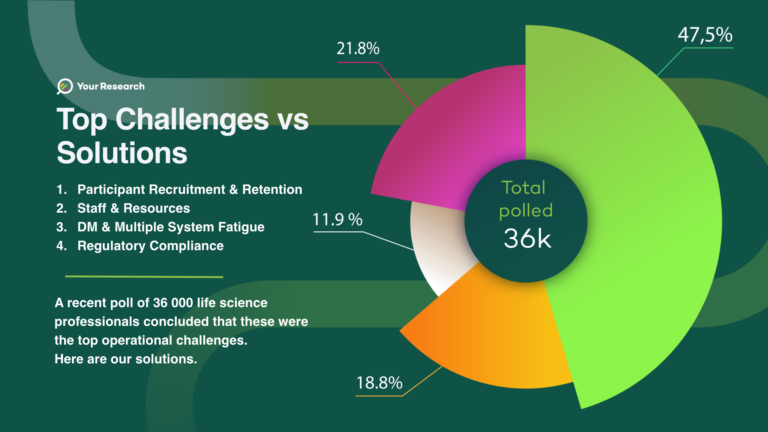
Top Challenges in Clinical Trial Operations - Your Research

Datacapt All in one Electronic Data Collection Software
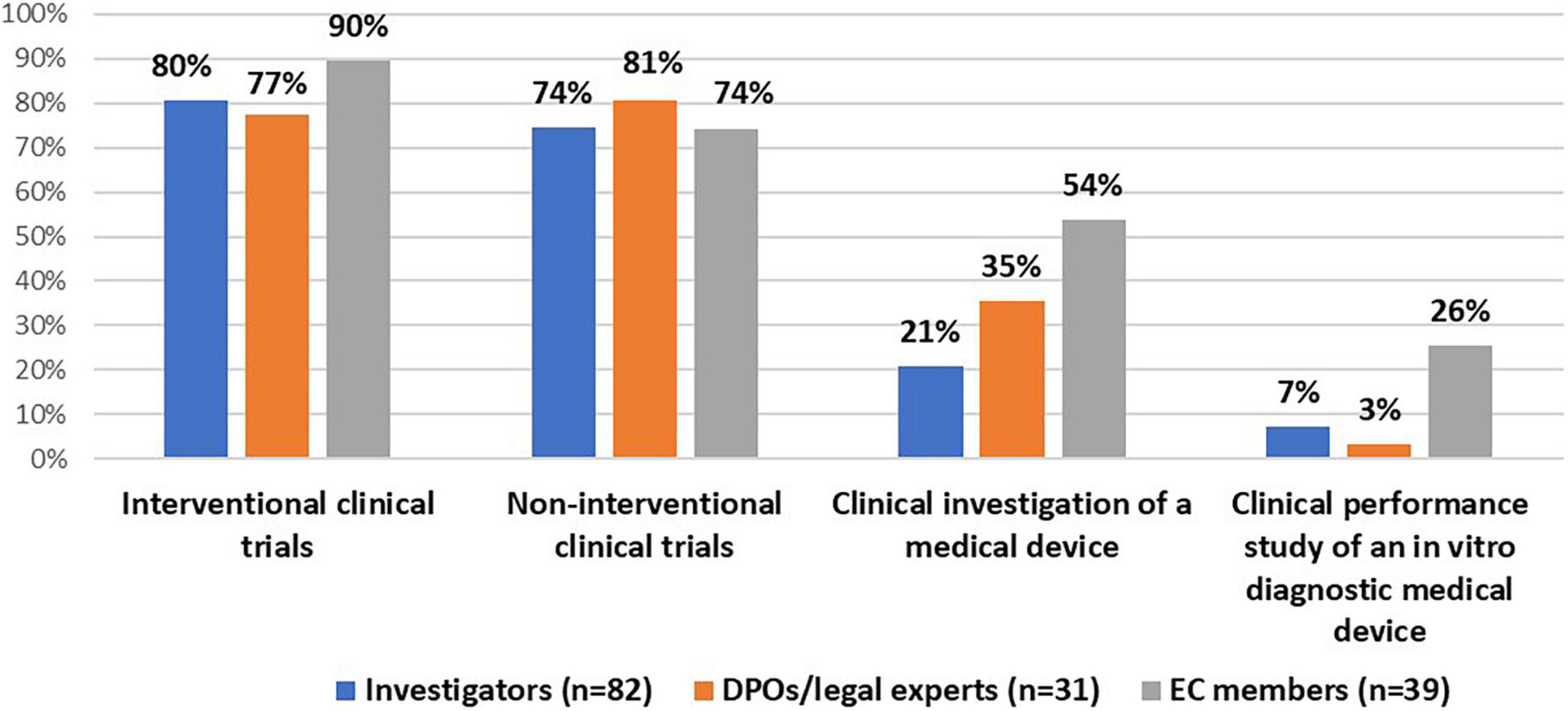
Frontiers Rethinking informed consent in the time of COVID-19
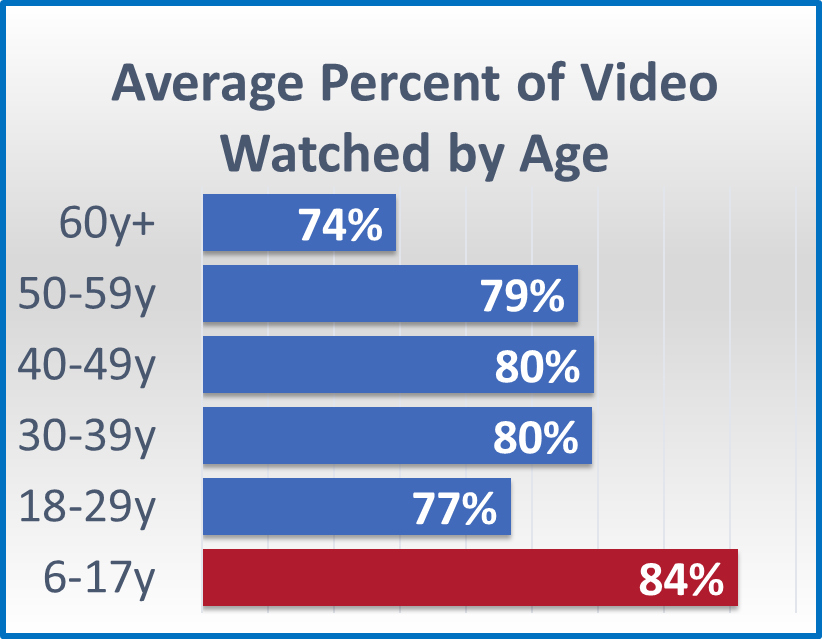
eConsent In Clinical Trials Insights For Implementation During

8 Clinical Trial Trends in 2023
de
por adulto (o preço varia de acordo com o tamanho do grupo)