ANANDA Scientific Announces FDA approval of the IND for the Clinical Trial on the Treatment of Opioid Use Disorder (OUD)
Por um escritor misterioso
Descrição
ANANDA Scientific Inc., (a biotech pharma company) today announced approval by the U.S. Food and Drug Administration (FDA) of the Investigational New

Ananda Scientific (@AnandaScience) / X

40th Annual Meeting of the Society for Medical Decision Making Montréal, Québec, Canada, October 13–17, 2018, 2019

ANANDA Scientific announces FDA approval of the IND for the clinical trial on the treatment of Opioid Use Disorder (OUD) - ANTARA News

A Decade of FDA-Approved Drugs (2010–2019): Trends and Future Directions

ANANDA Scientific Announces FDA approval of the IND for the Clinical Trial on the Treatment of Opioid Use Disorder (OUD)

40th Annual Meeting of the Society for Medical Decision Making Montréal, Québec, Canada, October 13–17, 2018, 2019

ANANDA Scientific and David Geffen School of Medicine UCLA Announce Clinical Trial Utilizing Liquid StructureTM Cannabidiol (CBD) for the Treatment of Opioid Use Disorder (OUD)

Pharmaceutical – Ananda Scientific

Justin Molignoni CRNP on LinkedIn: ANANDA Scientific Announces FDA approval of the IND for the Clinical Trial…
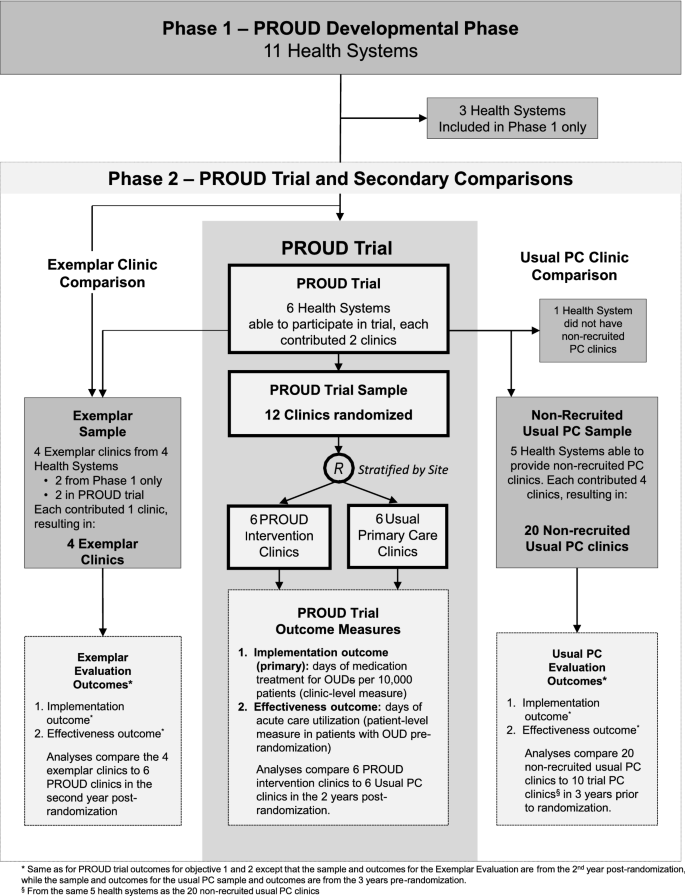
PRimary Care Opioid Use Disorders treatment (PROUD) trial protocol: a pragmatic, cluster-randomized implementation trial in primary care for opioid use disorder treatment, Addiction Science & Clinical Practice

Blog Articles And Insights
de
por adulto (o preço varia de acordo com o tamanho do grupo)






