Arsenic Trioxide Induces Apoptosis of Human Monocytes during Macrophagic Differentiation through Nuclear Factor-κB-Related Survival Pathway Down-Regulation
Por um escritor misterioso
Descrição
Arsenic trioxide (As2O3) is known to be toxic toward leukemia cells. In this study, we determined its effects on survival of human monocytic cells during macrophagic differentiation, an important biological process involved in the immune response. As2O3 used at clinically relevant pharmacological concentrations induced marked apoptosis of human blood monocytes during differentiation with either granulocyte-macrophage colony-stimulating factor or macrophage colony-stimulating factor. Apoptosis of monocytes was associated with increased caspase activities and decreased DNA binding of p65 nuclear factor-κB (NF-κB); like As2O3, the selective NF-κB inhibitor ( E )-3-[(4-methylphenyl)-sulfonyl]-2-propenenitrile (Bay 11-7082) strongly reduced survival of differentiating monocytes. The role of NF-κB in arsenic toxicity was also studied in promonocytic U937 cells during phorbol 12-myristate 13-acetate-induced macrophagic differentiation. In these cells, As2O3 first reduced DNA binding of p65 NF-κB and subsequently induced apoptosis. In addition, overexpression of the p65 NF-κB subunit, following stable infection with a p65 retroviral expressing vector, increased survival of As2O3-treated U937 cells. As2O3 specifically decreased protein levels of X-linked inhibitor of apoptosis protein and FLICE-inhibitory protein, two NF-κB-regulated genes in both U937 cells and blood monocytes during their differentiations. Finally, As2O3 was found to inhibit macrophagic differentiation of monocytic cells when used at cytotoxic concentrations; however, overexpression of the p65 NF-κB subunit in U937 cells reduced its effects toward differentiation. In contrast to monocytes, well differentiated macrophages were resistant to low concentrations of As2O3. Altogether, our study demonstrates that clinically relevant concentrations of As2O3 induced marked apoptosis of monocytic cells during in vitro macrophagic differentiation likely through inhibition of NF-κB-related survival pathways.
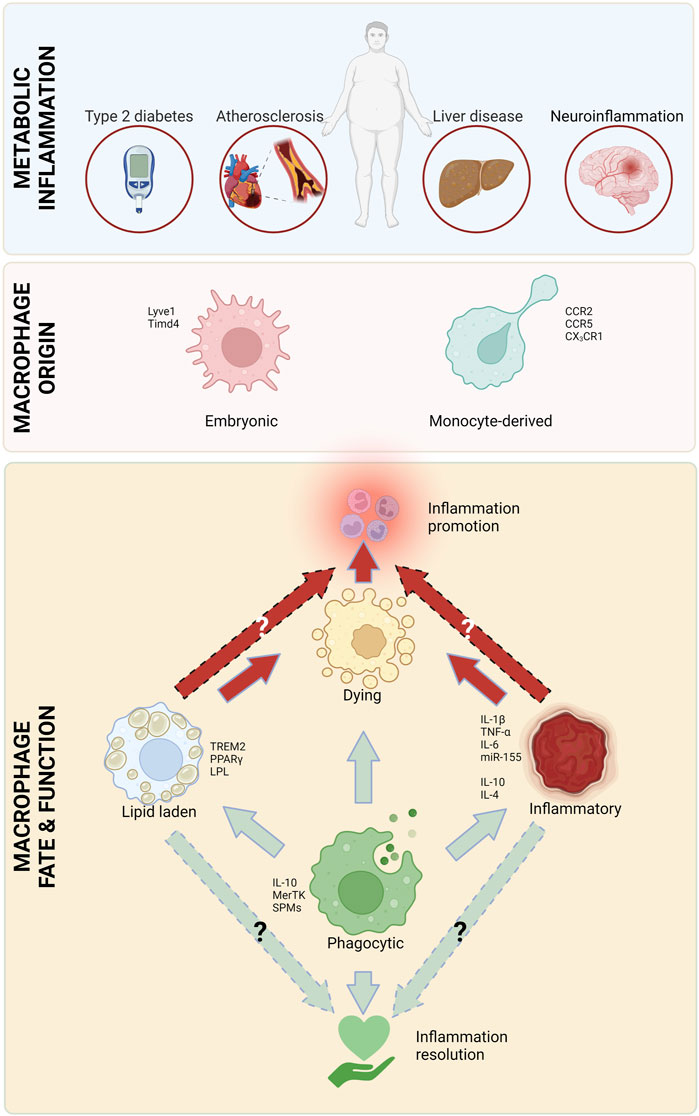
Frontiers The diverse roles of macrophages in metabolic inflammation and its resolution
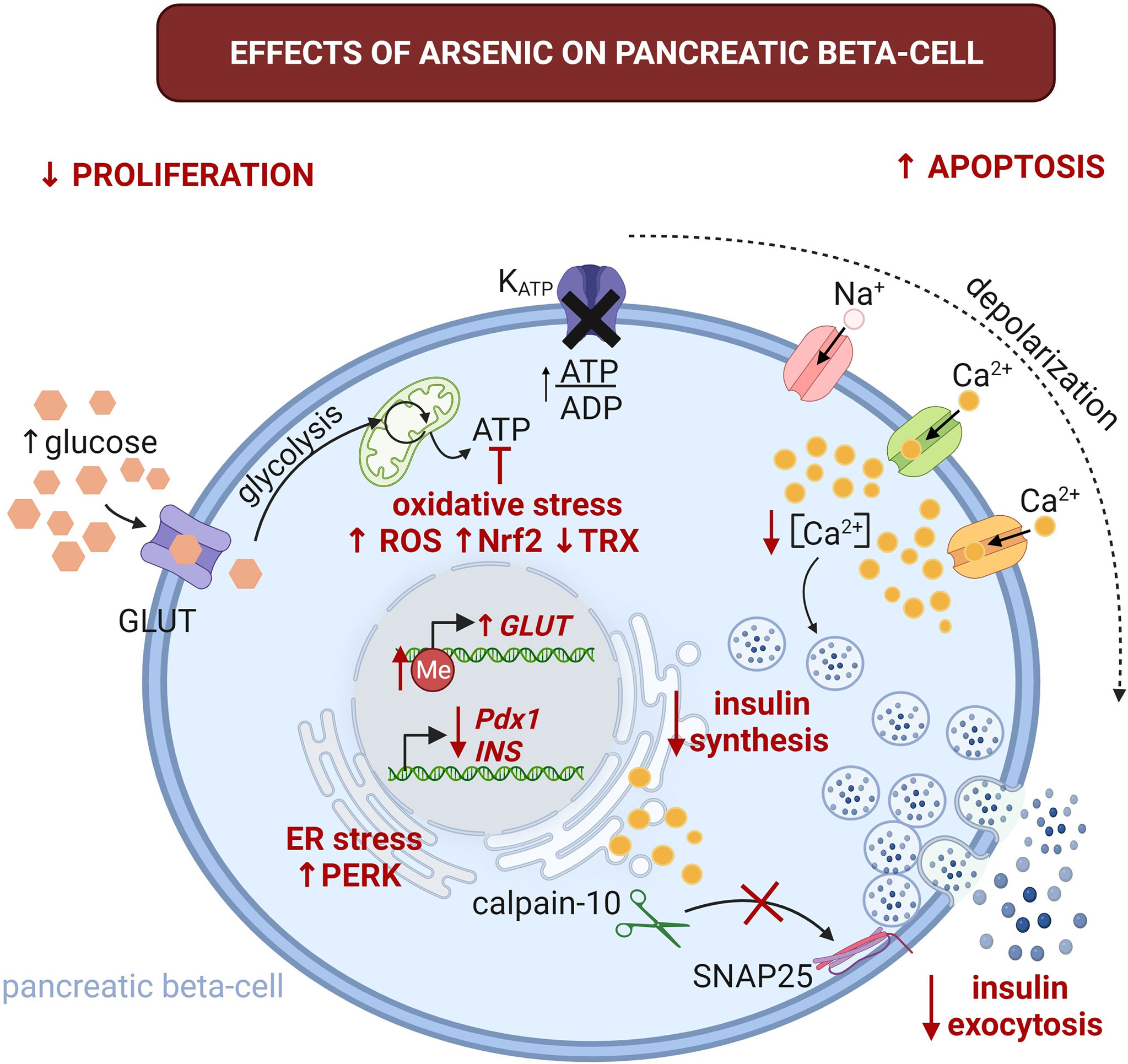
Frontiers Is Arsenic Exposure a Risk Factor for Metabolic Syndrome? A Review of the Potential Mechanisms

The Role of microRNAs in Arsenic-Induced Human Diseases: A Review
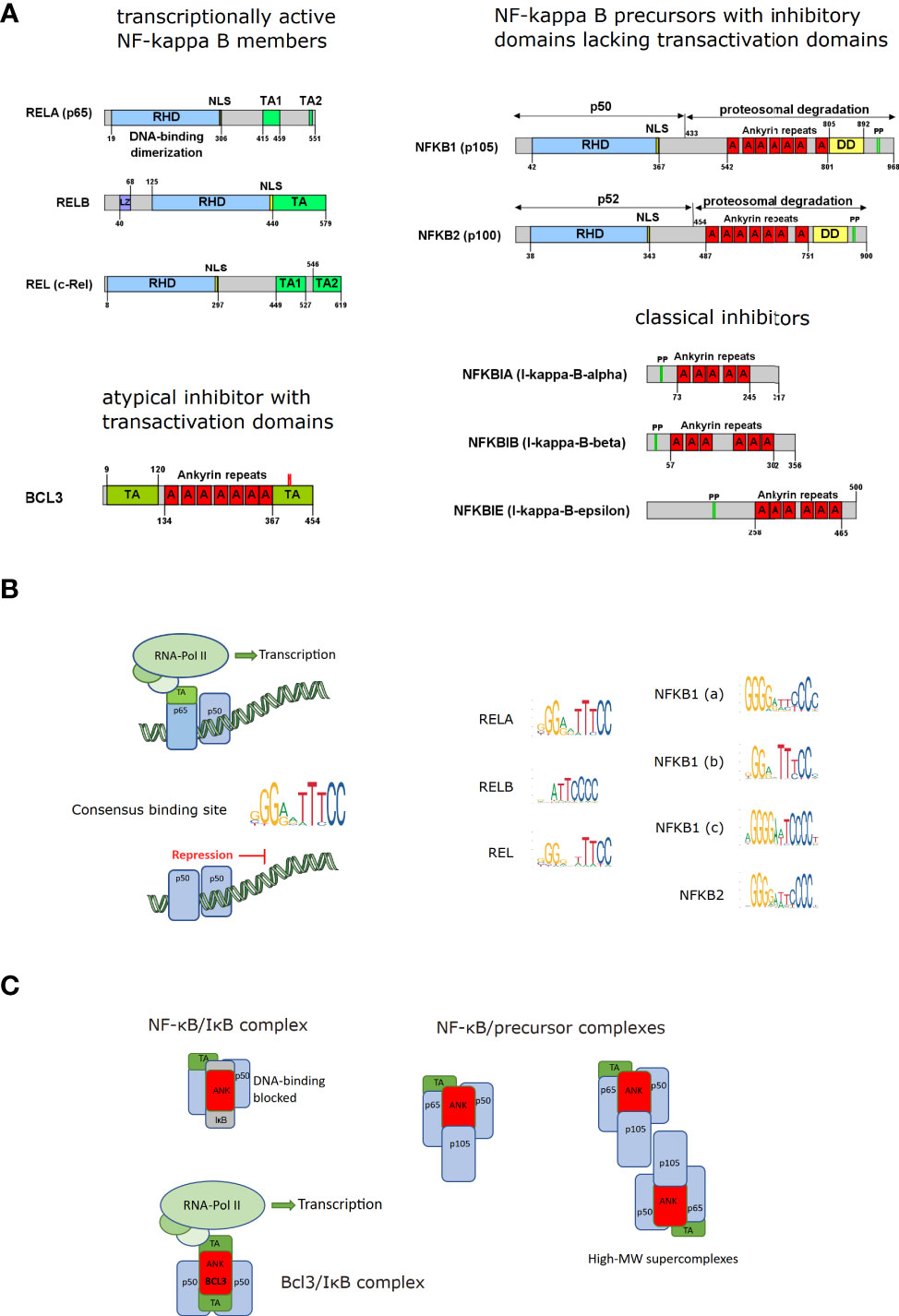
Frontiers NF-κB in monocytes and macrophages – an inflammatory master regulator in multitalented immune cells
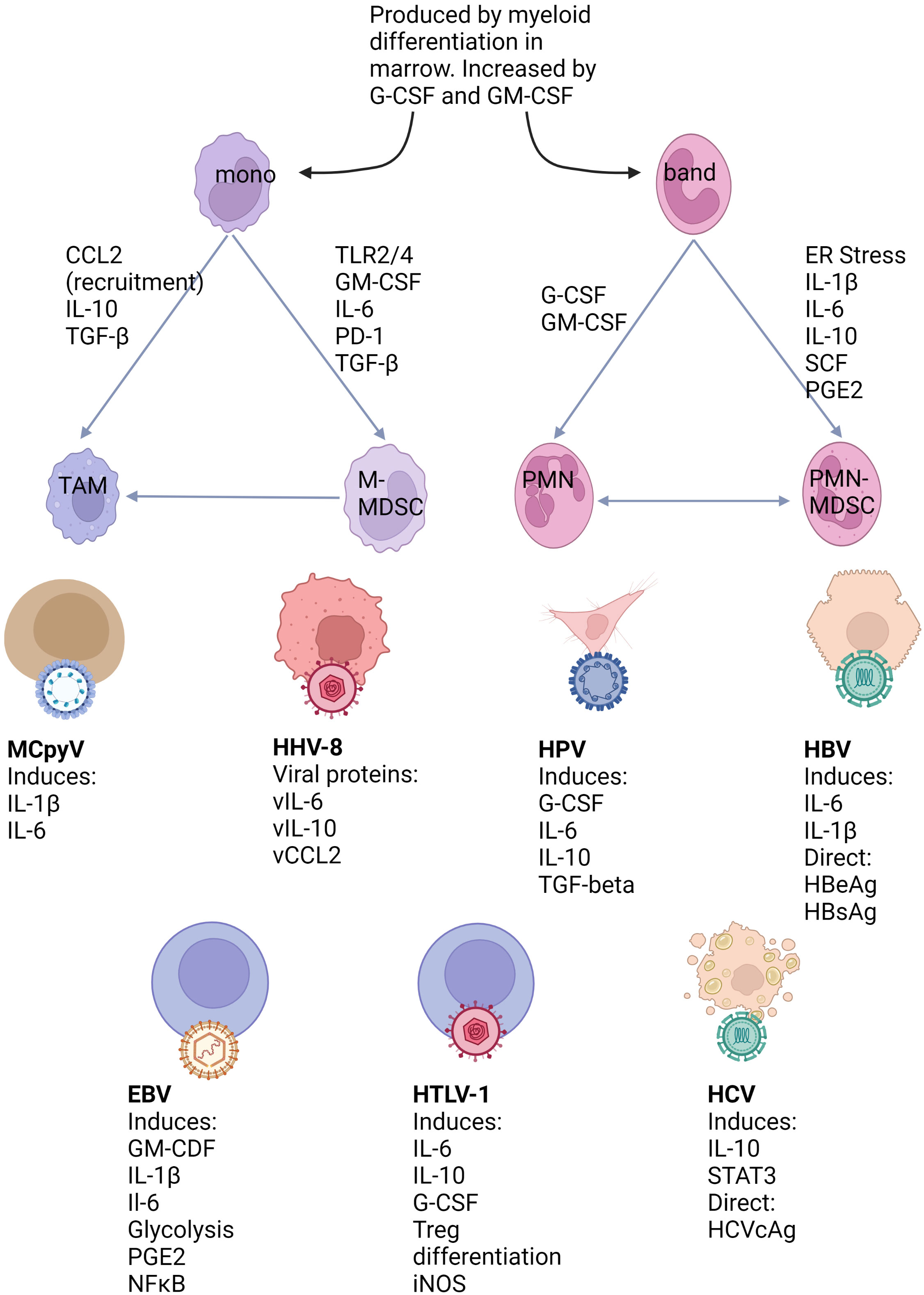
Frontiers Deciphering the roles of myeloid derived suppressor cells in viral oncogenesis

Down-regulation of RdRp complex and activated immune response due to increased arsenic level leads to decreased corona virus replication - ScienceDirect

miRs regulate inflammation, oxidative stress, apoptosis, and
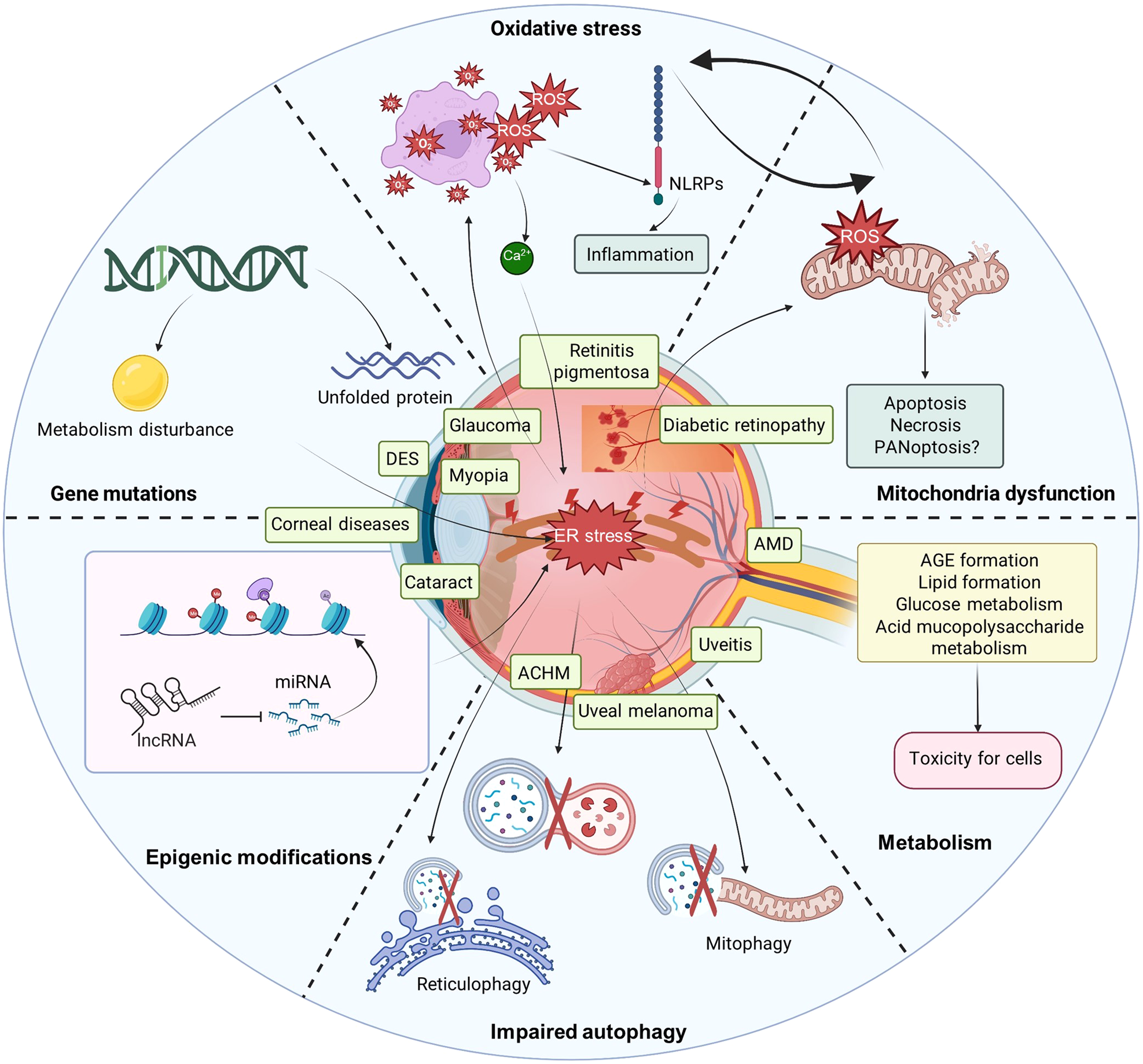
Endoplasmic reticulum stress: molecular mechanism and therapeutic targets

Macrophages in oxidative stress and models to evaluate the antioxidant function of dietary natural compounds - ScienceDirect

Phytochemicals targeting NF-κB signaling: Potential anti-cancer interventions - ScienceDirect
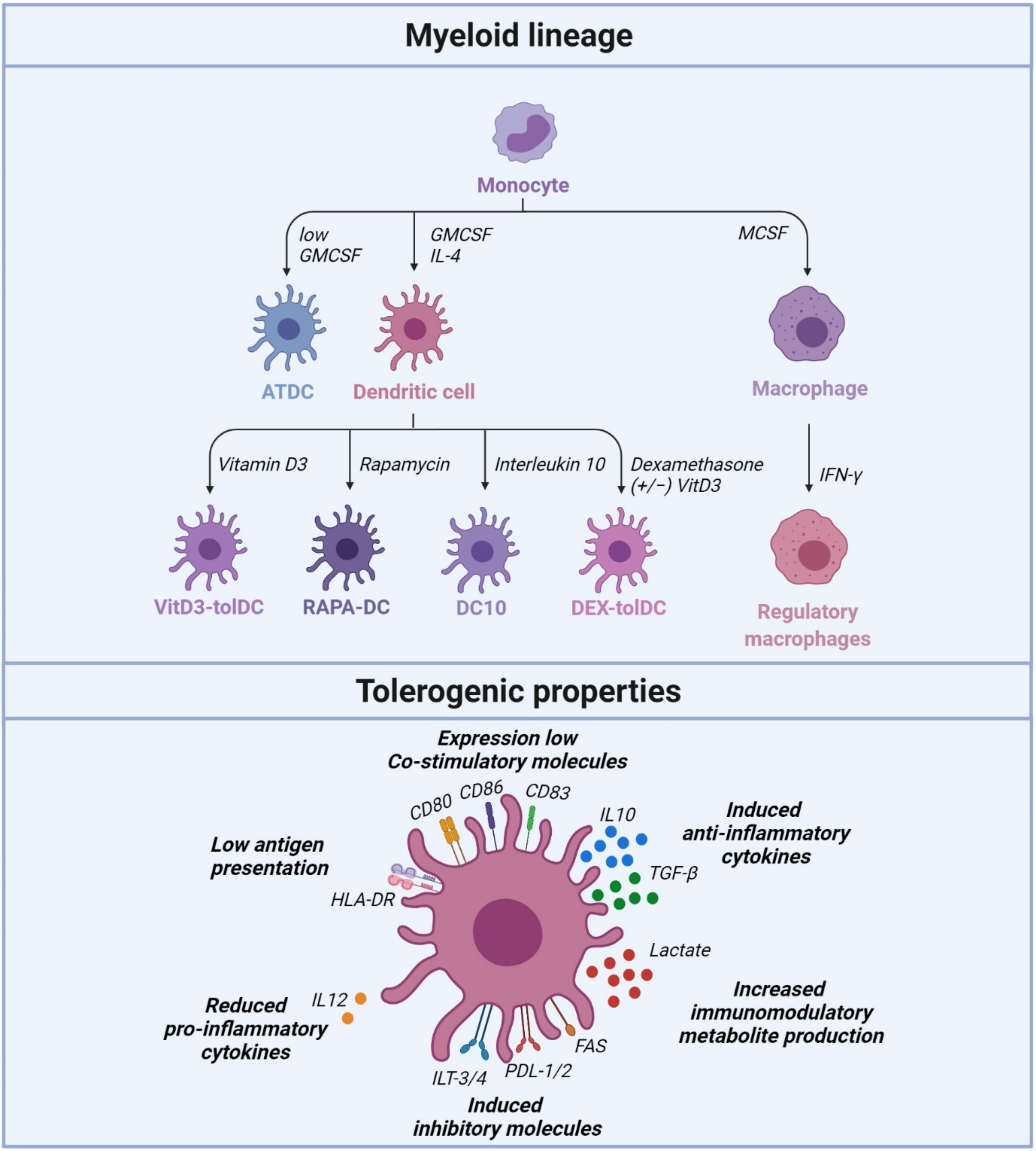
IJMS, Free Full-Text
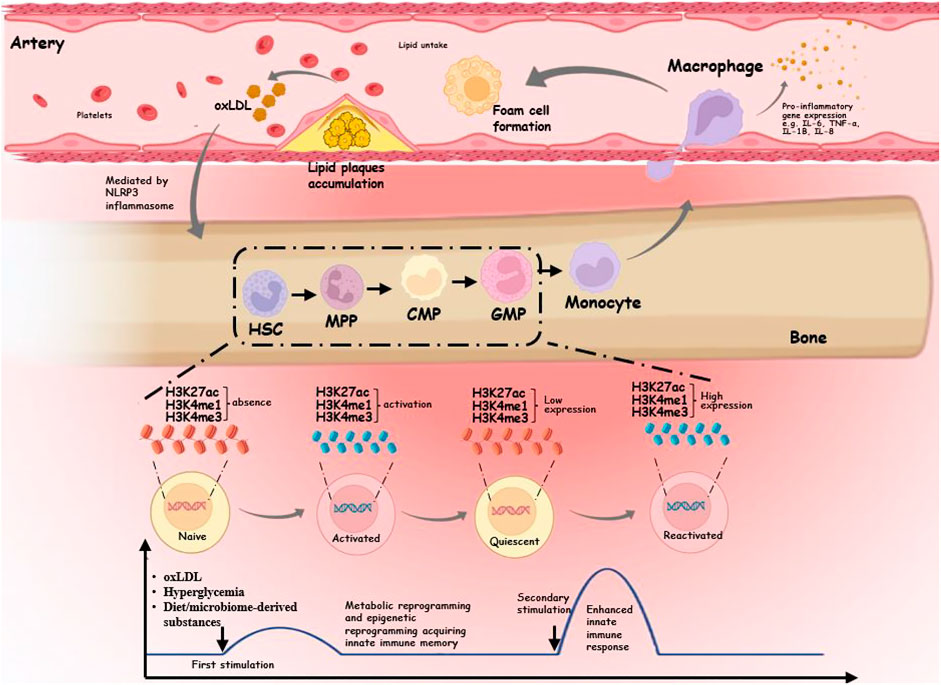
Frontiers Trained immunity in monocyte/macrophage: Novel mechanism of phytochemicals in the treatment of atherosclerotic cardiovascular disease

The multiple roles of salt-inducible kinases in regulating physiology
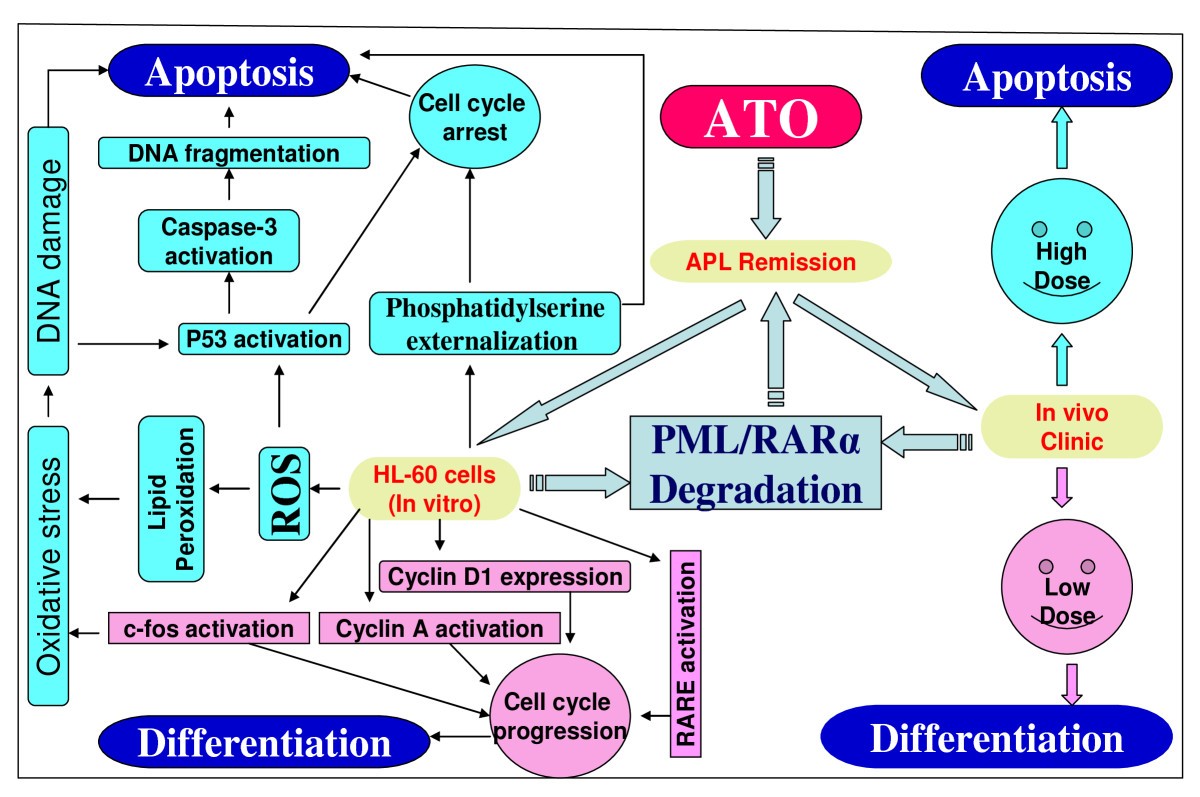
Basic Mechanisms of Arsenic Trioxide (ATO)-Induced Apoptosis in Human Leukemia (HL-60) Cells, Journal of Hematology & Oncology
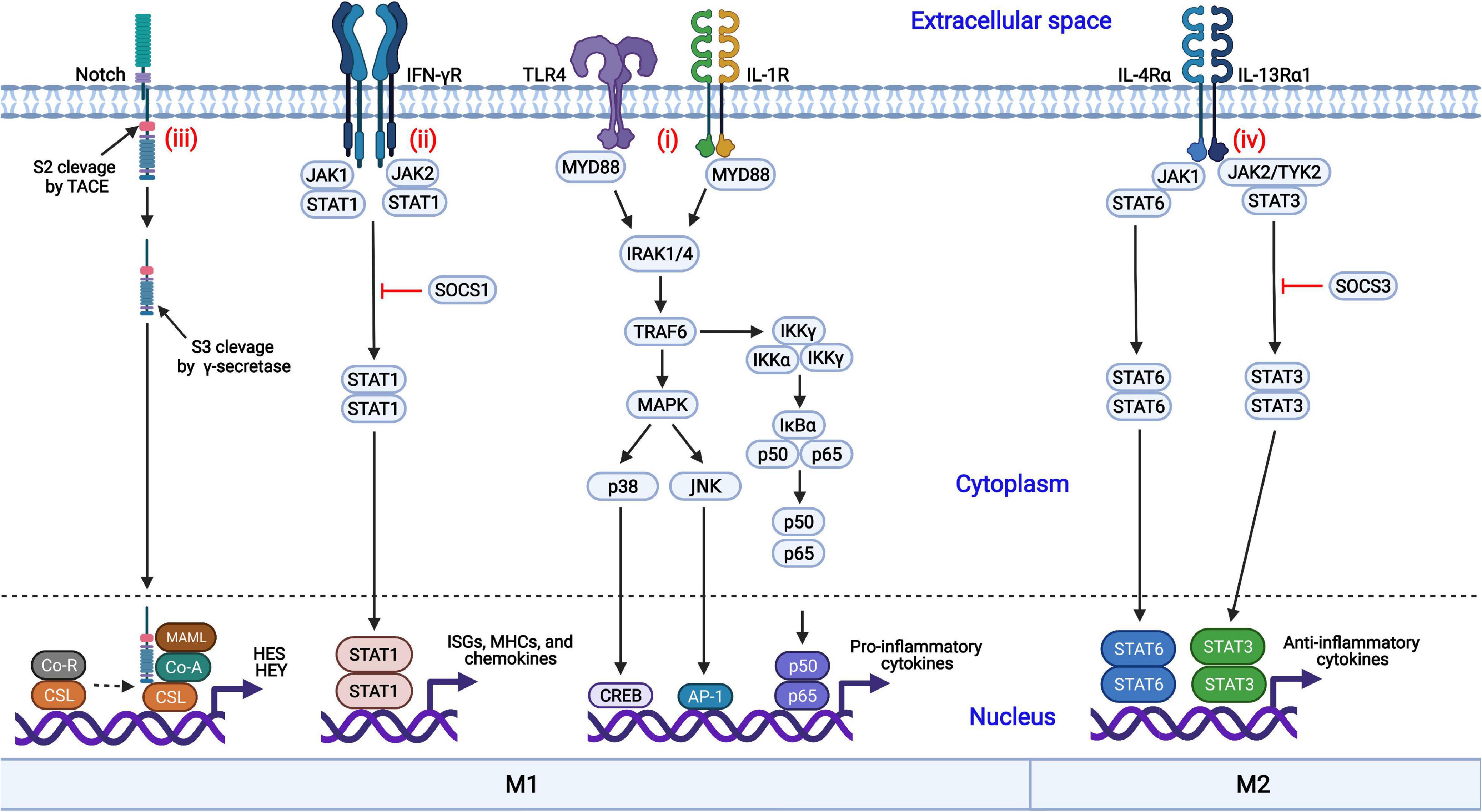
Frontiers Modulation of Macrophage Polarization by Viruses: Turning Off/On Host Antiviral Responses
de
por adulto (o preço varia de acordo com o tamanho do grupo)