GHGH Formula - C14H26O11 - Over 100 million chemical compounds
Por um escritor misterioso
Descrição
GHGH contains total 51 atom(s); 26 Hydrogen atom(s), 14 Carbon atom(s), and 11 Oxygen atom(s). Learn more about GHGH chemical formula at Mol-Instincts.

a. Suggest possible molecular formulas for a compound that h
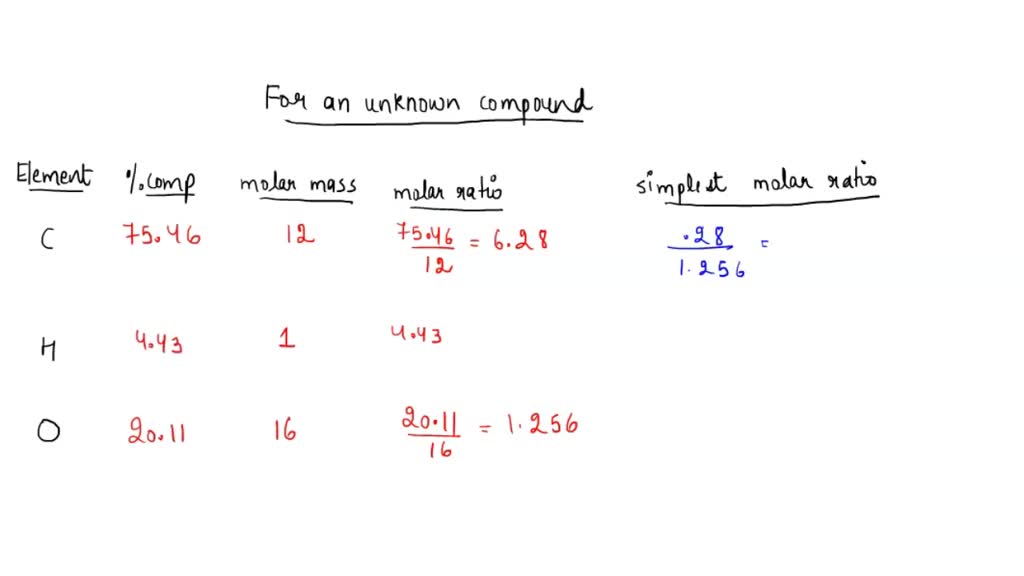
SOLVED: An unknown compound contains 75.46% Carbon, 4.43% Hydrogen, and 20.11% Oxygen by mass. The molecular mass is 318.31 g/mol. What is the molecular formula of the unknown compound?
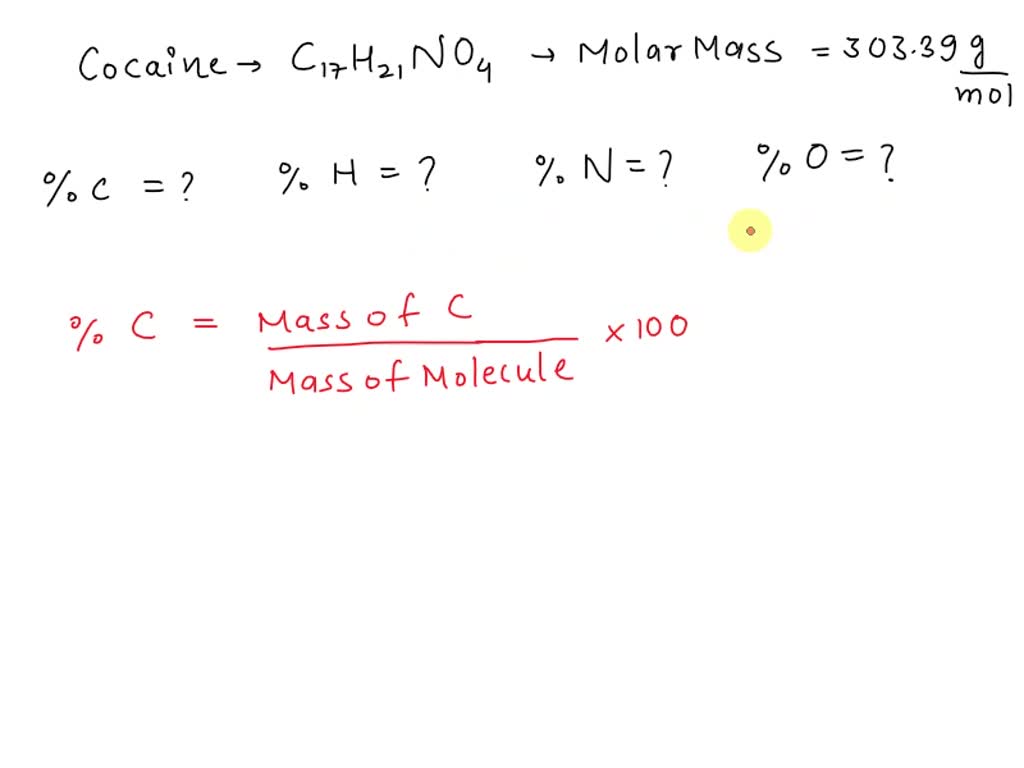
SOLVED: ***** The illegal drug cocaine has the chemical formula C17H21NO4 . Calculate the percent composition of each of the elements in the compound. (a) %C = (b) %H = (c) %N = (d) %O =

GHGH Formula - C14H26O11 - Over 100 million chemical compounds
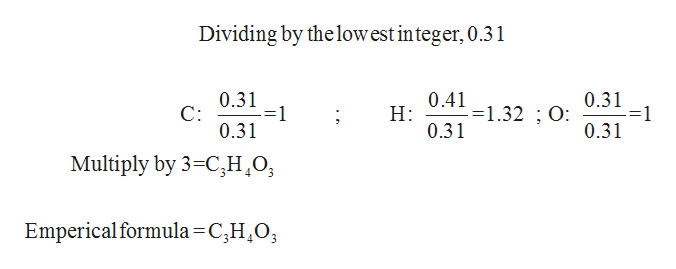
Answered: 9.00 g of a certain Compound X, known…

Solved The empirical formula of a certain compound is CHN.

Human growth hormone (32-38) Formula - C39H60N8O13 - Over 100 million chemical compounds
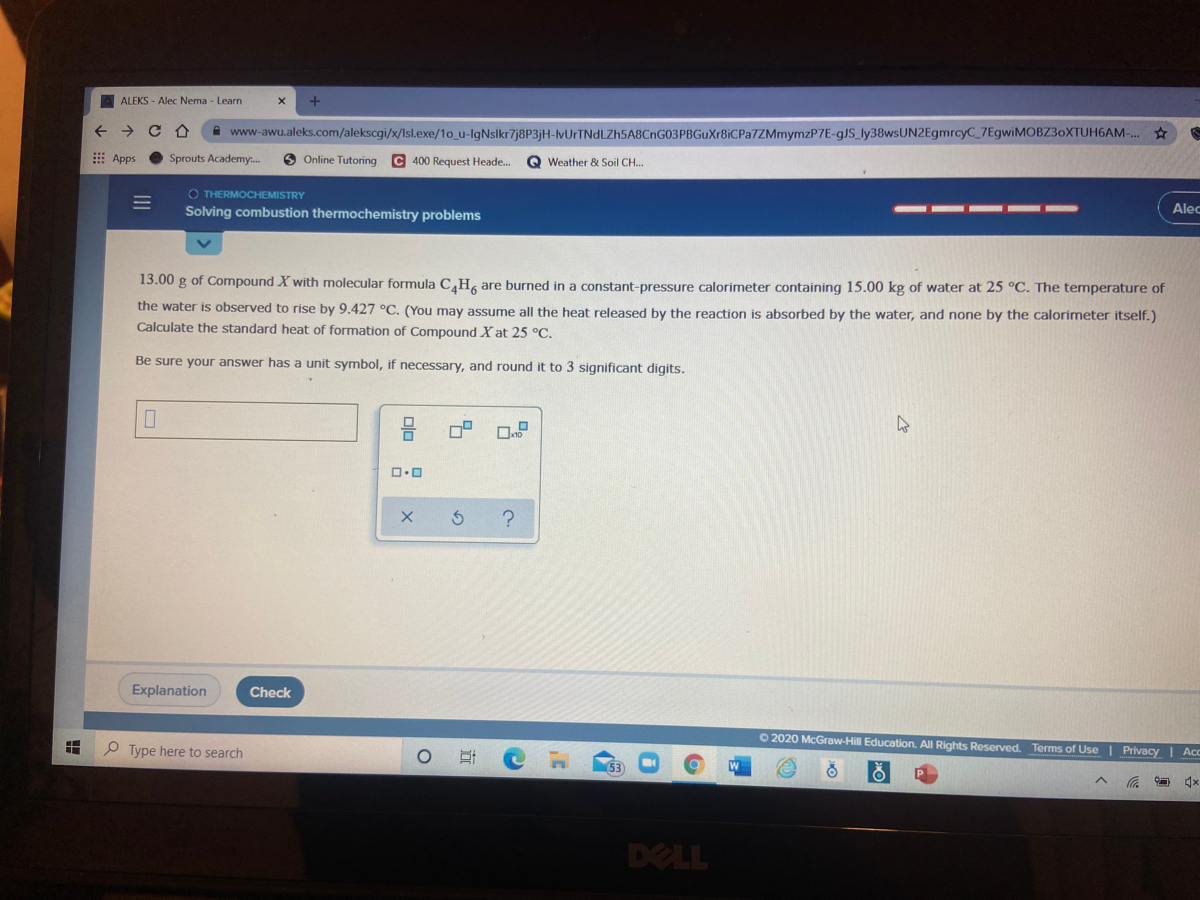
Answered: 13.00 g of Compound X with molecular…
A compound is found to contain 39.99 % carbon, 6.727 % hydrogen, and 53.28 % oxygen by weight. To answer

4-Heptanone SDF/Mol File - C7H14O - Over 100 million chemical compounds

Solved 7. Compound EE, CsH100 gives a positive result
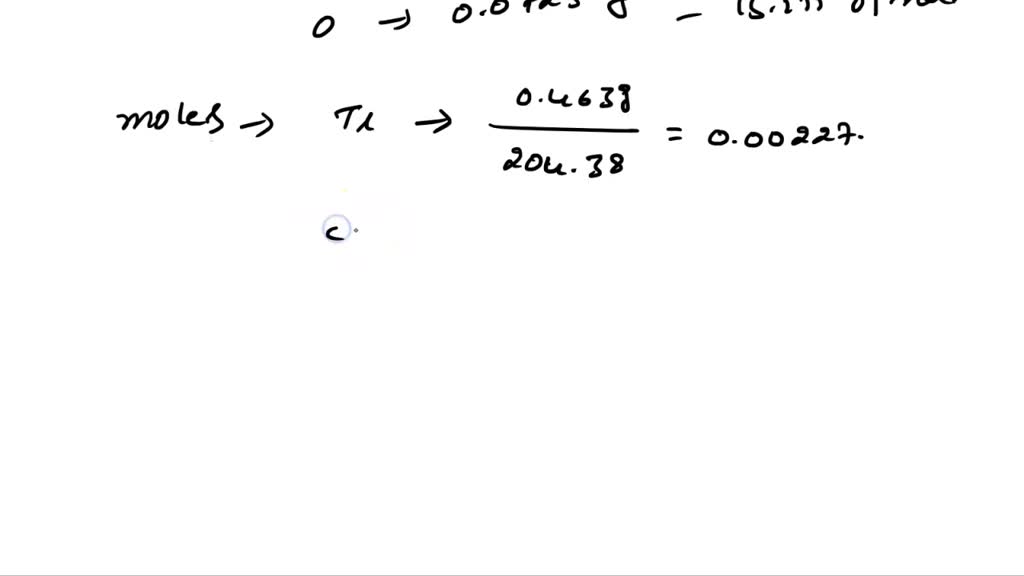
SOLVED: NAME the compound which contains 0.463 g Tl (#81) 0.0544 g of carbon, 0.00685 g of Hydrogen and 0.0725 g oxygen by finding its empirical formula: (1 pt) Draw a molecular
de
por adulto (o preço varia de acordo com o tamanho do grupo)







/i.s3.glbimg.com/v1/AUTH_bc8228b6673f488aa253bbcb03c80ec5/internal_photos/bs/2021/r/D/1i2pogQNqjBCePEdKGlQ/066-dppi-40421059-004.jpg)