NA Labetalol Hydrochloride Impurity - Anax Laboratories
Por um escritor misterioso
Descrição
Anax Laboratories provides Chemical industry users with Impurities of Labetalol Hydrochloride Impurity(NA) Boiling point Melting point, Labetalol Hydrochloride Impurity (NA ) Density MSDS Formula Use,If You also need to Labetalol Hydrochloride Impurity (NA ) Other information,welcome to contact us.
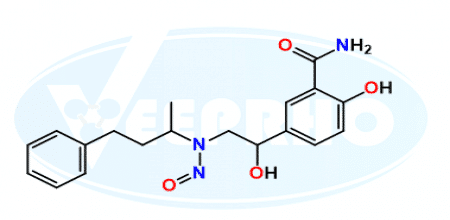
2820170-74-7: N-Nitroso Labetalol - Veeprho

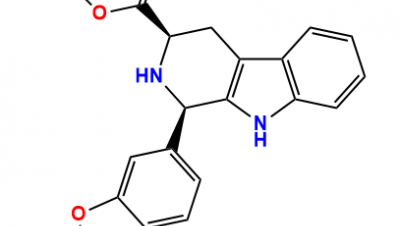
Structures of labetalol hydrochloride and its related impurity
Impurities Index, CAS Number, Product Code

TODO ORO BAJA CALIFORNIA 5
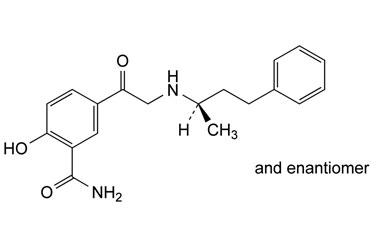
Labetalol Hydrochloride-impurities

PDF) Determination of alcuronium dichloride in plasma by high-performance liquid chromatography without solvent extraction

126110-99-4 Fosinopril Sodium Impurity - Anax Laboratories

LABETALOL HYDROCHLORIDE INJECTION, USP

Amneal Pharmaceuticals, Inc. - Amneal Pharmaceuticals LLC Issues Voluntary Nationwide Recall of Metformin Hydrochloride Extended Release Tablets, USP, 500 mg and 750 mg, due to Detection of N-Nitrosodimethylamine (NDMA) Impurity to Consumer Level

logo.jpg
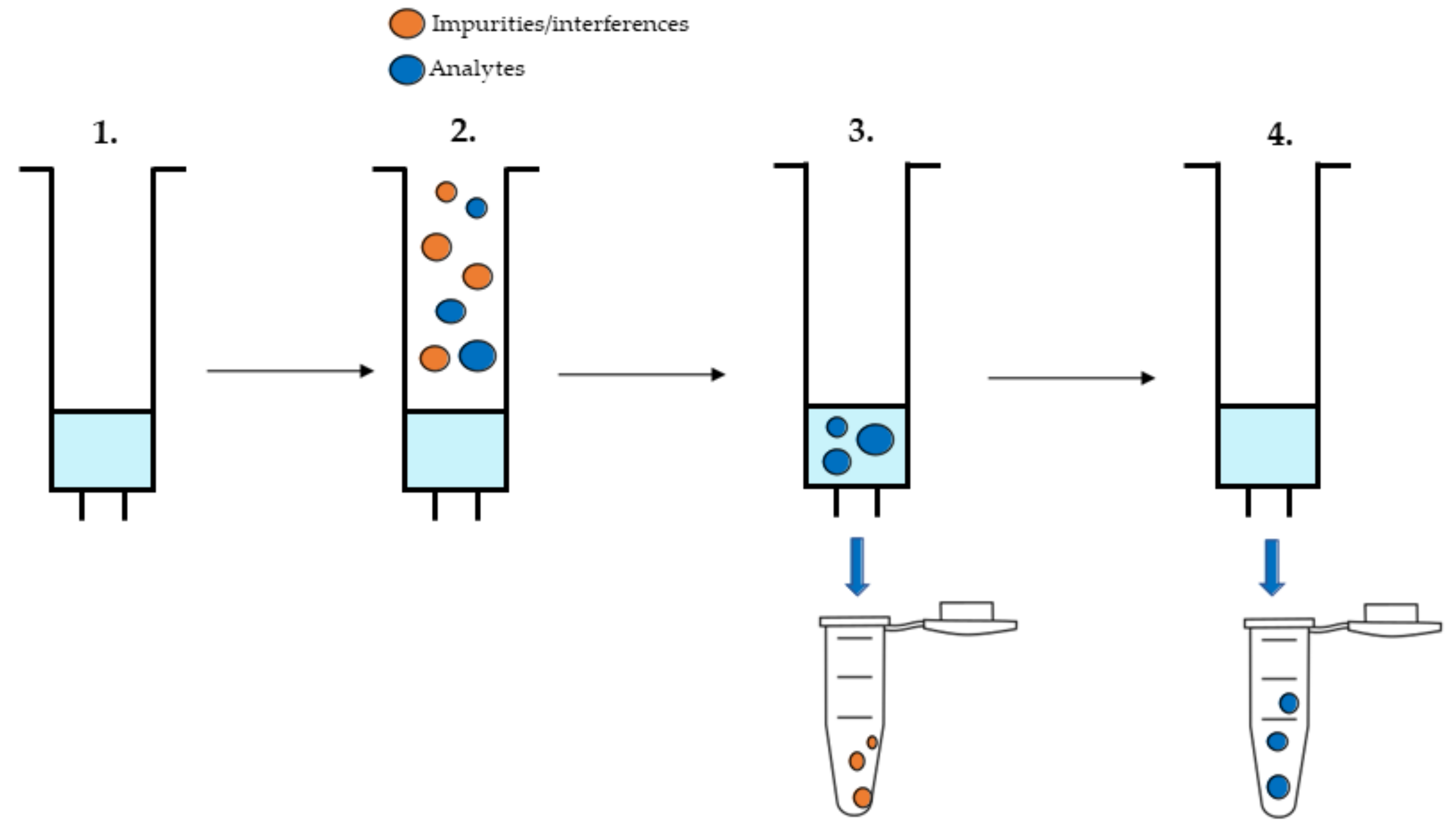
Separations, Free Full-Text
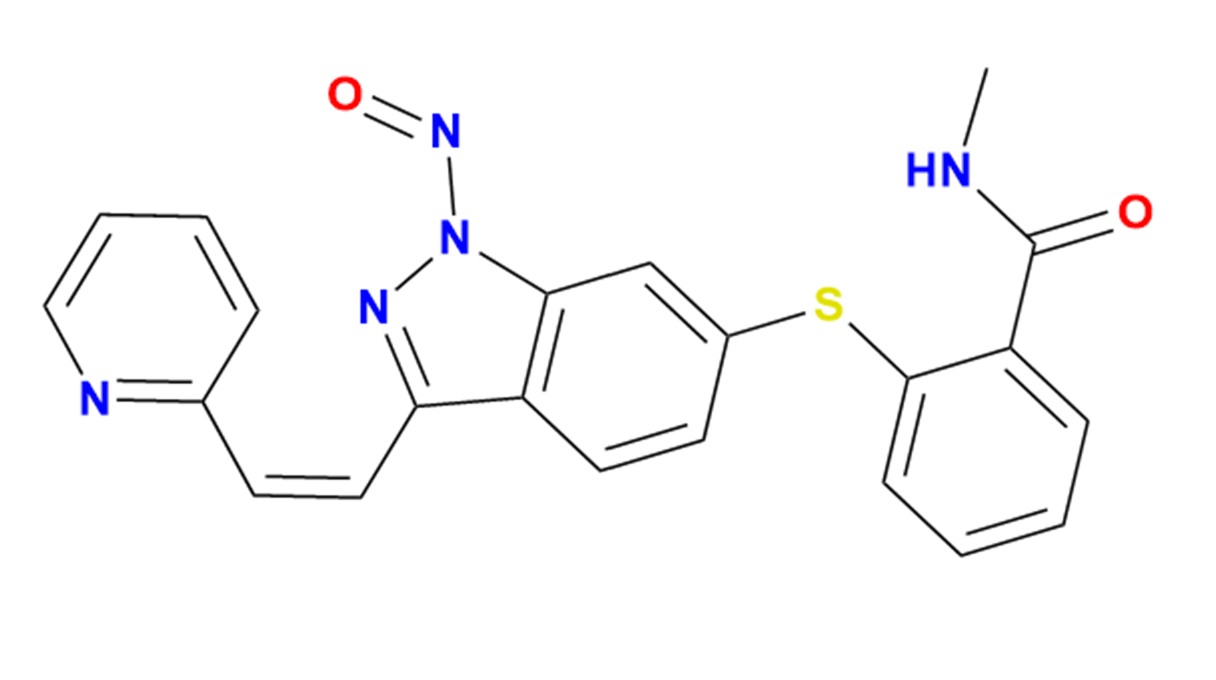
NA Axitinib Nitroso Impurity - Anax Laboratories
de
por adulto (o preço varia de acordo com o tamanho do grupo)