Validation of an anti-α-Gal IgE fluoroenzyme-immunoassay for the screening of patients at risk of severe anaphylaxis to cetuximab, BMC Cancer
Por um escritor misterioso
Descrição
Background The link between immediate hypersensitivity reactions (HSR) following the first cetuximab infusion and the IgE sensitization against anti-galactose-α-1,3-galactose (α-Gal) is now well-established. An automated Fluoroenzyme-Immunoassay (FEIA) is available and may facilitate the screening of patients with anti-α-Gal IgE before treatment. Methods This study aimed to evaluate its performances as compared to a previously validated anti-cetuximab IgE ELISA, using 185 samples from two previously studied cohorts. Results Despite 21.1% of discrepancies between the two techniques, FEIA discriminated better positive patients and similarly negative ones with a ≥ 0.525 kUA/L threshold. Sensitivity was 87.5% for both tests, specificity was better for FEIA (96.3% vs ELISA: 82.1%). FEIA had a higher positive likelihood ratio (23.9 vs ELISA: 4.89) and a similar negative likelihood ratio (0.13 vs ELISA: 0.15). In our population, the risk of severe HSR following a positive test was higher with FEIA (56.7% vs ELISA: 19.6%) and similar following a negative test (0.7% vs ELISA: 0.8%). Conclusion Although the predictive value of the IgE screening before cetuximab infusion remains discussed, this automated commercial test can identify high-risk patients and is suitable for routine use in laboratories. It could help avoiding cetuximab-induced HSR by a systematic anti-α-Gal IgE screening before treatment.

Alpha-Gal-containing biologics and anaphylaxis - ScienceDirect

Full article: Current and Future Strategies for the Diagnosis and Treatment of the Alpha-Gal Syndrome (AGS)
Forest plot and SROC curve. Download Scientific Diagram

Study schema showing the 545 cetuximab-treated patients from whom

Risk of bias and applicability concerns summary.

Forest plot and SROC curve. Download Scientific Diagram

Prevalence of anti-cetuximab IgE. IgE levels were measured in serum

PDF) Case Report About Fatal or Near-Fatal Hypersensitivity Reactions to Cetuximab: Anticetuximab IgE as a Valuable Screening Test

Description of the study population. HSR: Hypersensitivity reaction.

PDF) Anti-cetuximab IgE ELISA for identification of patients at high risk of cetuximab-induced anaphylaxis
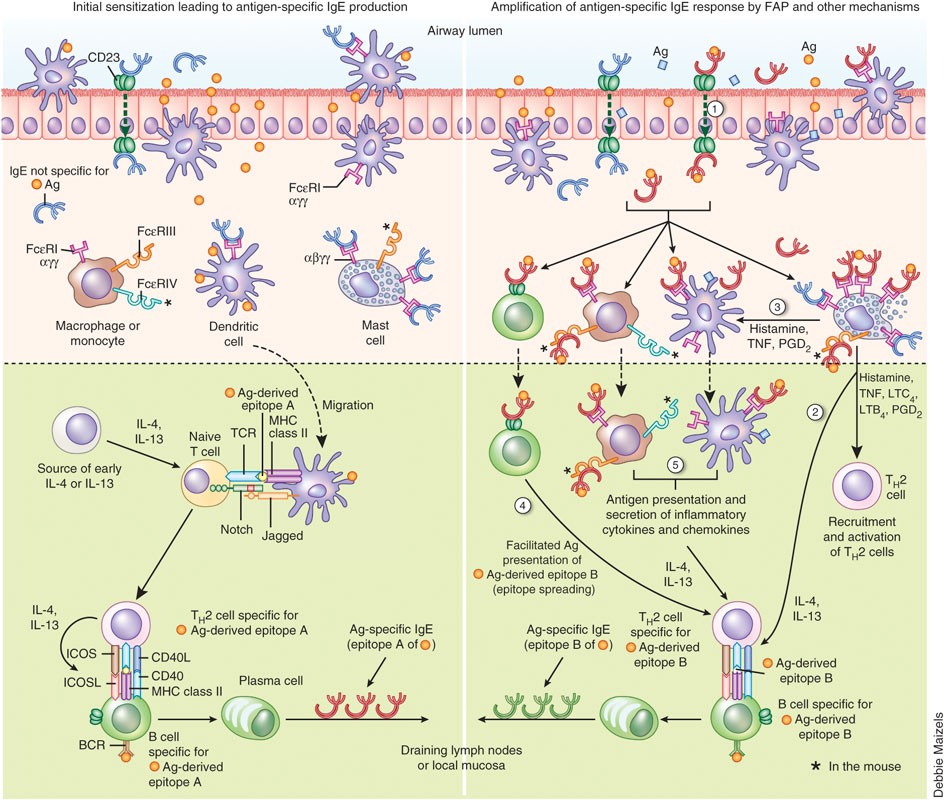
IgE and mast cells in allergic disease

The basophil activation test differentiates between patients with alpha-gal syndrome and asymptomatic alpha-gal sensitization - ScienceDirect

IJMS, Free Full-Text

Cetuximab-Induced Anaphylaxis and IgE Specific for Galactose-α-1,3-Galactose

The future of food allergy: Challenging existing paradigms of clinical practice - Anagnostou - 2023 - Allergy - Wiley Online Library
de
por adulto (o preço varia de acordo com o tamanho do grupo)







