A. Mean ASDAS and B. mean BASDAI to week 96. Safety set (N = 89).
Por um escritor misterioso
Descrição

Reduction of anterior uveitis flares in patients with axial spondyloarthritis on certolizumab pegol treatment: final 2-year results from the multicenter phase IV C-VIEW study - Irene E. van der Horst-Bruinsma, Rianne E.

Full article: Impact of different types of exercise programs on ankylosing spondylitis: a systematic review and meta-analysis
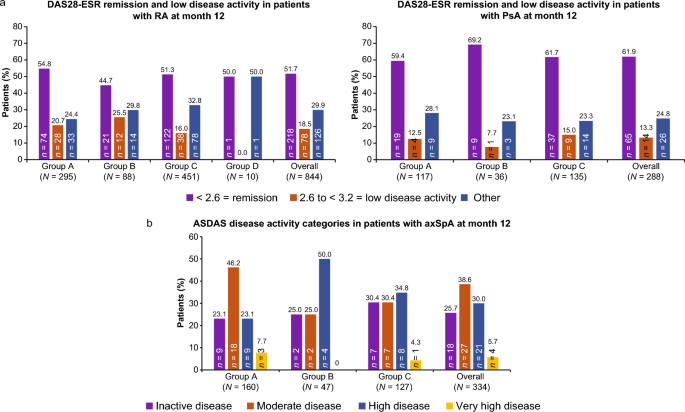
Real-World Effectiveness and Safety of SDZ ETN, an Etanercept Biosimilar, in Patients with Rheumatic Diseases: Final Results from Multi-Country COMPACT Study

ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update
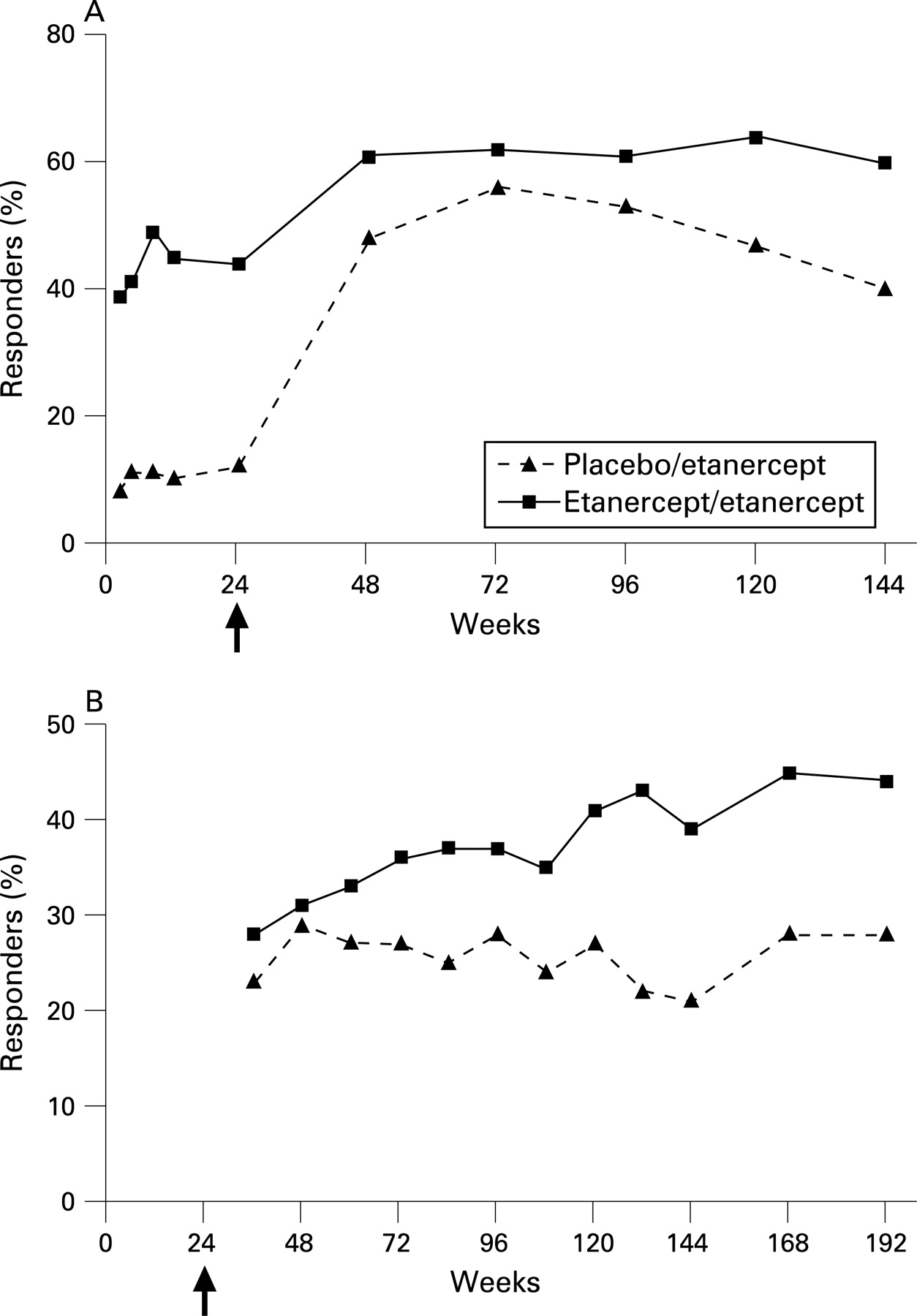
Efficacy and safety of up to 192 weeks of etanercept therapy in patients with ankylosing spondylitis

Axial spondyloarthritis - The Lancet

Spondyloarthritis in Colombia - Wilson Armando Bautista-Molano by Wilson Bautista-Molano - Issuu

Measures of symptoms and disease status in ankylosing spondylitis: Ankylosing Spondylitis Disease Activity Score (ASDAS), Ankylosing Spondylitis Quality of Life Scale (ASQoL), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing

TNF Inhibitor Therapy, Ankylosing Spondylitis
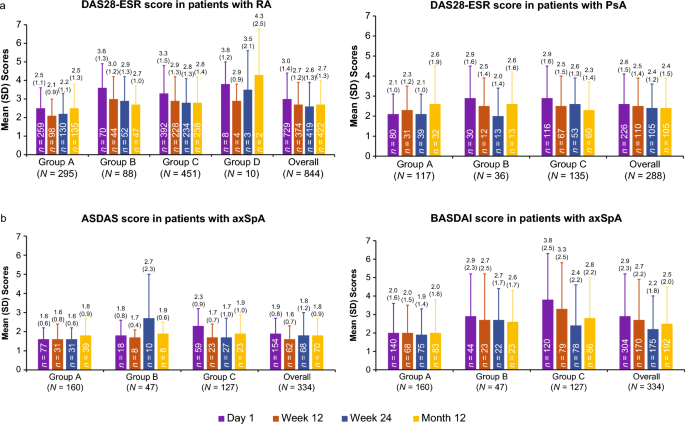
Real-World Effectiveness and Safety of SDZ ETN, an Etanercept Biosimilar, in Patients with Rheumatic Diseases: Final Results from Multi-Country COMPACT Study

These highlights do not include all the information needed to use CYLTEZO safely and effectively. See full prescribing information for CYLTEZO. CYLTEZO® (adalimumab-adbm) injection, for subcutaneous use Initial U.S. Approval: 2017 CYLTEZO (
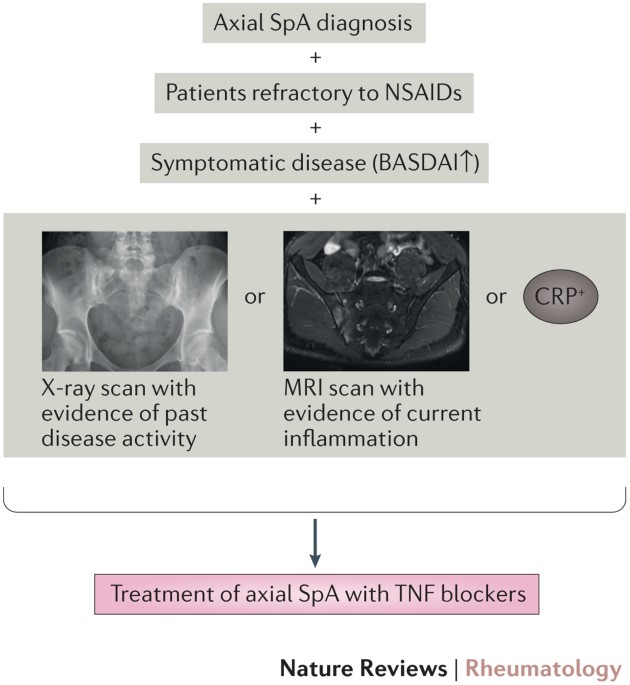
New evidence on the management of spondyloarthritis
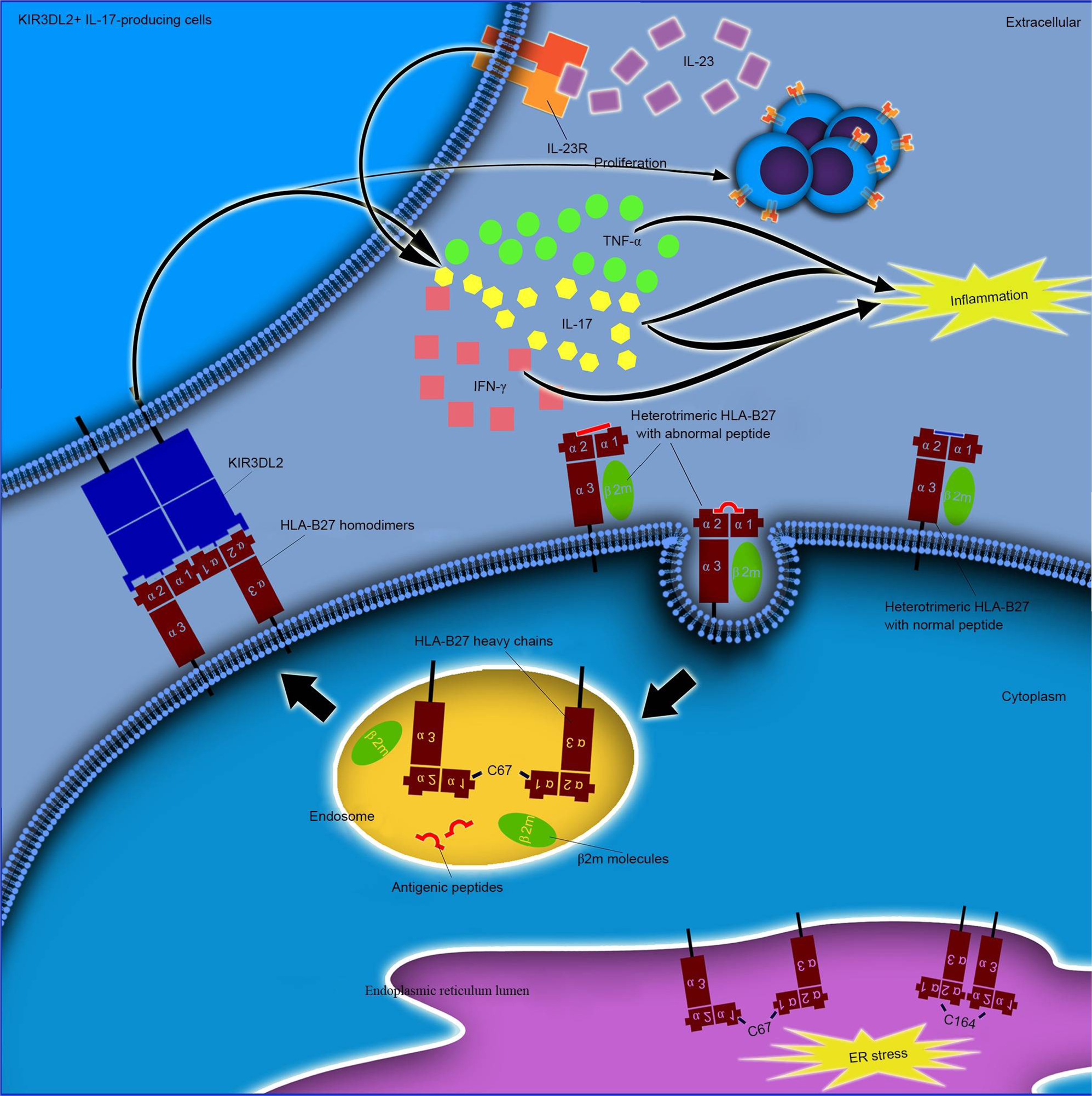
Ankylosing spondylitis: etiology, pathogenesis, and treatments
de
por adulto (o preço varia de acordo com o tamanho do grupo)