Rocket boosted by FDA alignment on pivotal gene therapy trial
Por um escritor misterioso
Descrição
Rocket Pharmaceuticals has reach | Rocket Pharmaceuticals has reached alignment with the FDA on the design of a pivotal phase 2 rare disease trial, positioning it to run a 12-patient study that could support accelerated approval of a gene therapy.

Rocket soars on stock offering, FDA alignment for gene therapy RP-A501

Current state of U.S. Food and Drug Administration regulation for cellular and gene therapy products: potential cures on the horizon - ScienceDirect

Heartpoint Global preparing to begin human trials for Intellistent
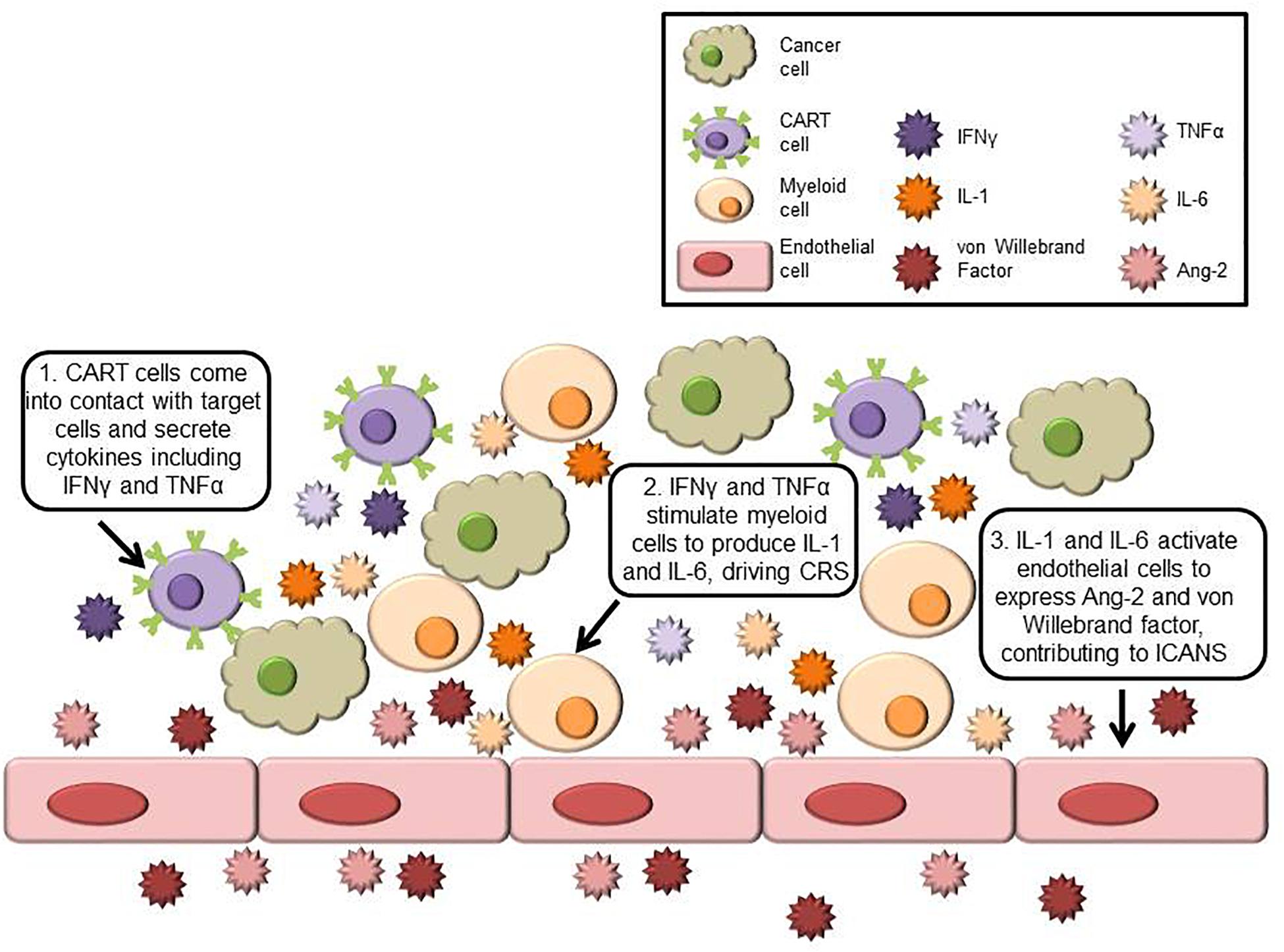
Frontiers Neurotoxicity and Cytokine Release Syndrome After Chimeric Antigen Receptor T Cell Therapy: Insights Into Mechanisms and Novel Therapies

Nanosilica: Recent Progress in Synthesis, Functionalization, Biocompatibility, and Biomedical Applications

Steve Blank Innovation and Entrepreneurship August 2013
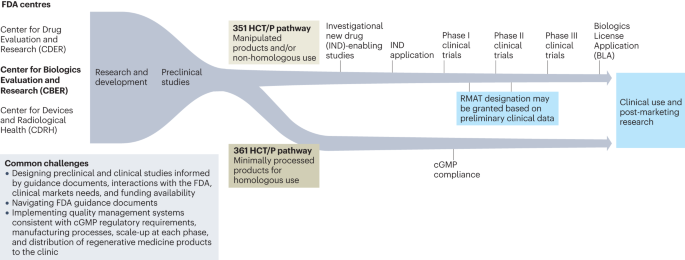
Commercialization of regenerative-medicine therapies
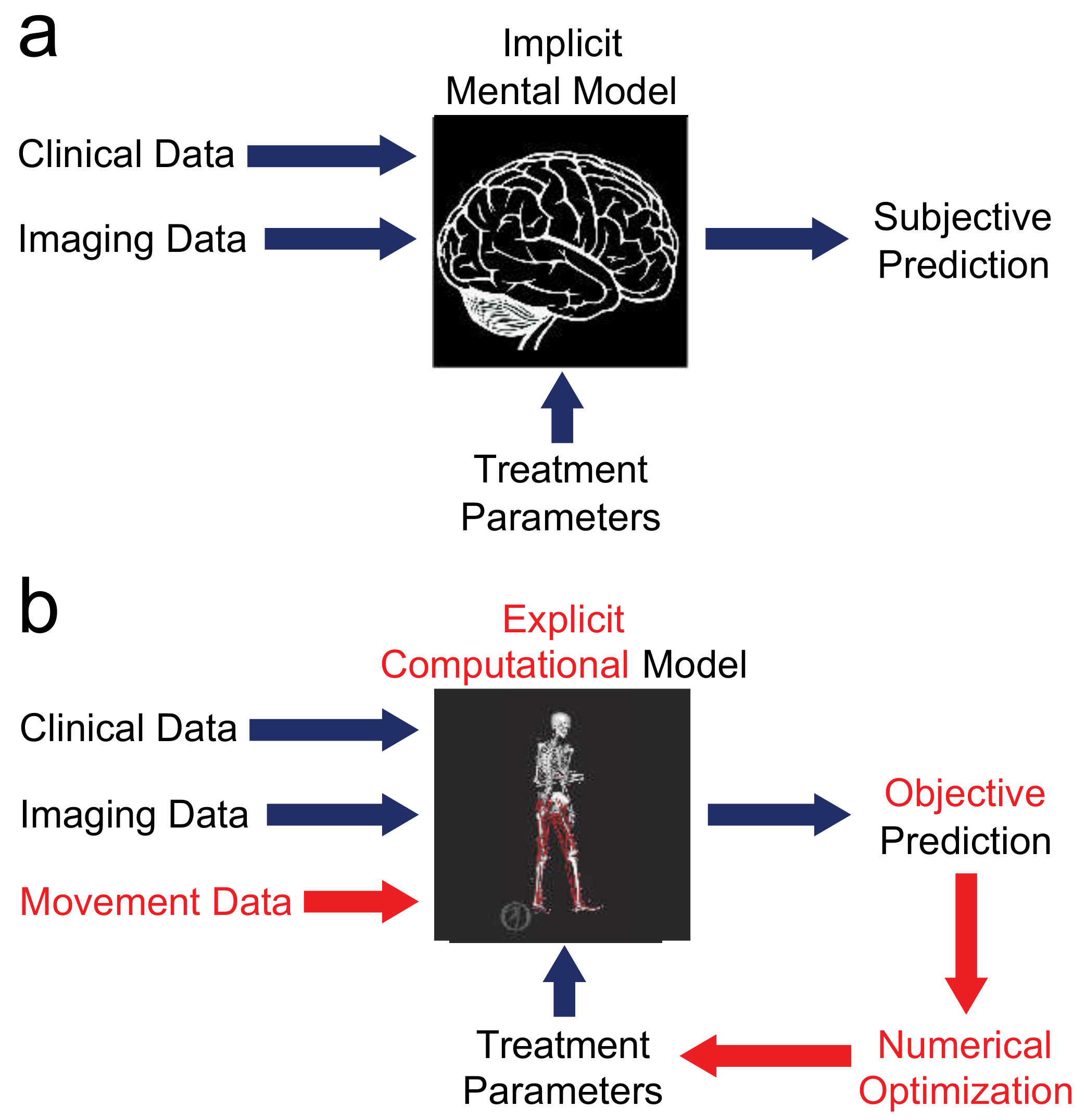
Applied Sciences, Free Full-Text

Raise the Line

Susan B. Nichols on LinkedIn: Rocket boosted as FDA alignment on pivotal gene therapy trial design sends…

Rocket receives show of confidence in gene therapy from FDA

Medical Micro/Nanorobots in Precision Medicine - Soto - 2020 - Advanced Science - Wiley Online Library

Safety and efficacy of gene replacement therapy for X-linked myotubular myopathy (ASPIRO): a multinational, open-label, dose-escalation trial - The Lancet Neurology

Rocket Pharmaceuticals Reaches FDA Alignment on Pivotal Phase 2 Trial Design for RP-A501 in Danon Disease
de
por adulto (o preço varia de acordo com o tamanho do grupo)







