hydrogen orbital wavefunction
Por um escritor misterioso
Descrição
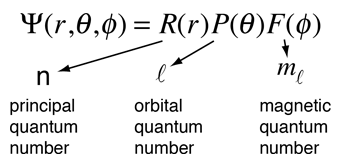
Hydrogen Schrodinger Equation
How to plot the hydrogen atom wave functions - Quora
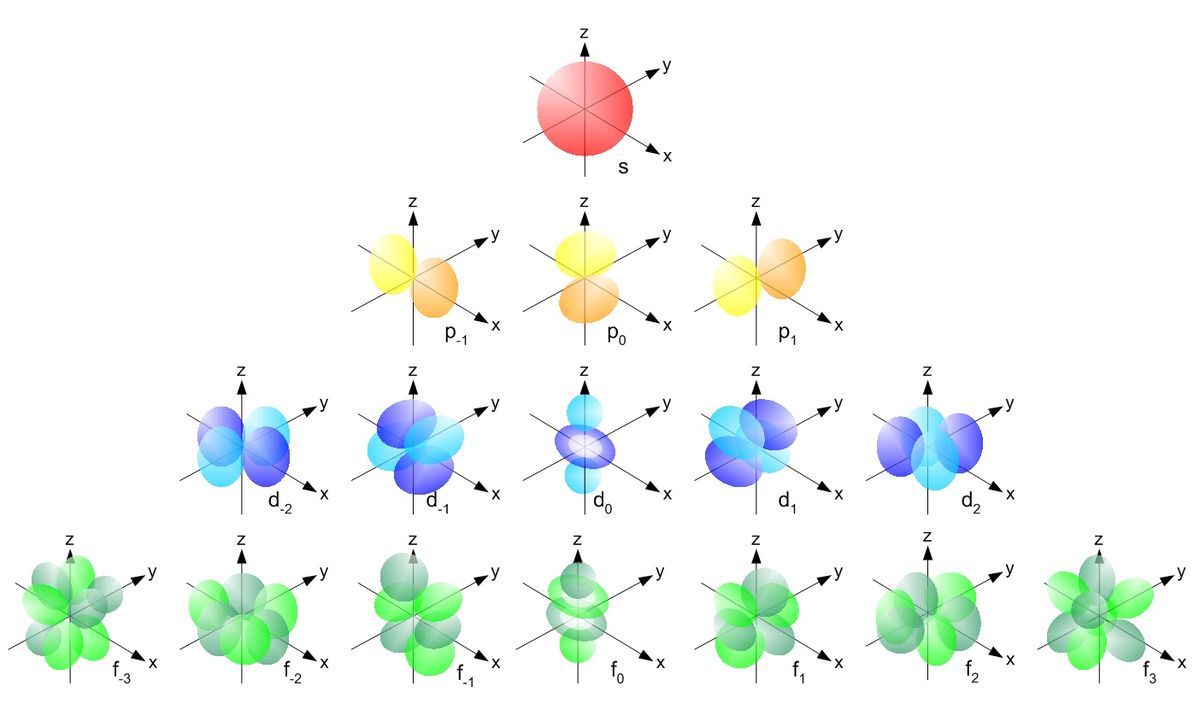
Hydrogen Atom Brilliant Math & Science Wiki
How were the shapes of s, p, d, and f orbitals determined? How did they get their names of s, p, d, and f?

quantum mechanics - Do the hydrogen atom's electron orbitals have Gaussian probability density functions? - Physics Stack Exchange

The Schrodinger wave equation hydrogen atom is: Psi_{2s}= dfrac{1}{4 sqrt{2 pi}} left( dfrac{1}{a_{0}} right)^{3/2} left( 2- dfrac{r_{0}}{a_{0}} right) e^{-dfrac{r_{0}}{a_{0}}}, where a_{0} is Bohr's radius. If the radial node is 2s be r_{0}
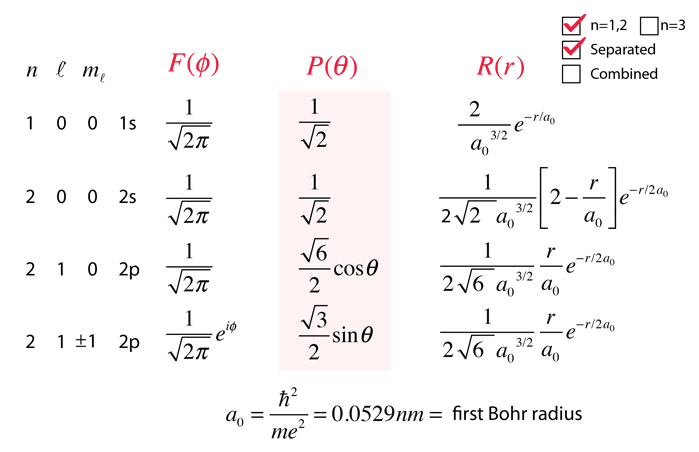
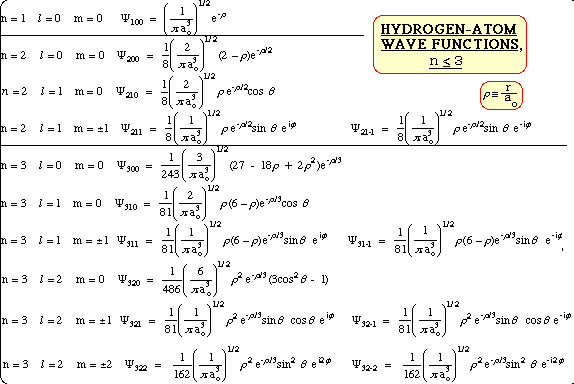
Hydrogen Wavefunctions
A poster that demonstrates the beauty of quantum chemistry using a classic example--the hydrogen electron wave function. Perfect for those
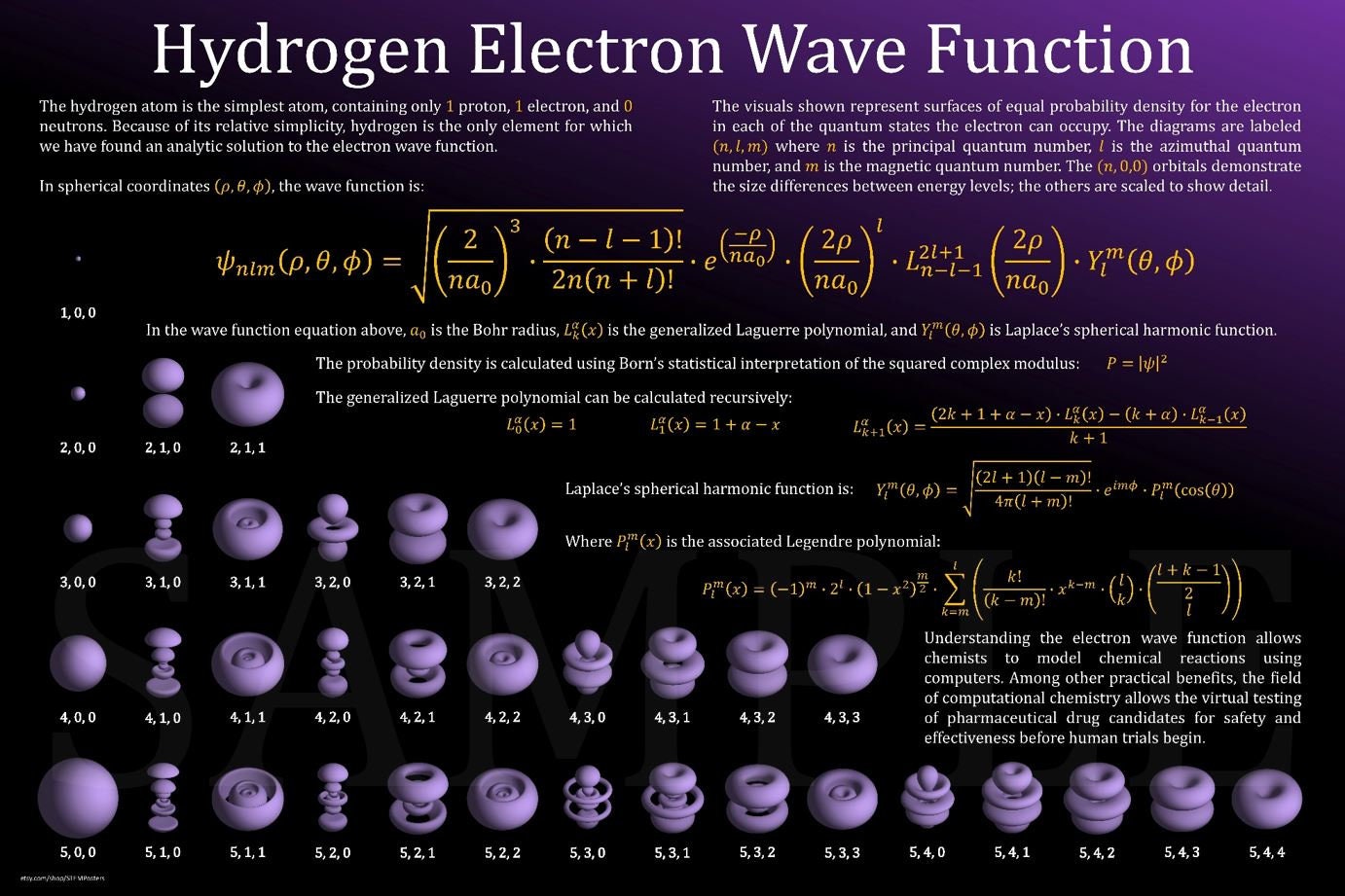
3D Hydrogen Electron Orbitals Poster
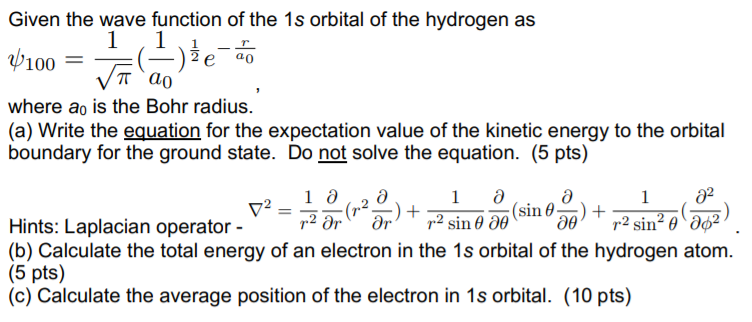
Solved Given the wave function of the 1s orbital of the
de
por adulto (o preço varia de acordo com o tamanho do grupo)







