ASAS-HI improvement ≥30%, ASDAS LDA status and ASAS40 response
Por um escritor misterioso
Descrição
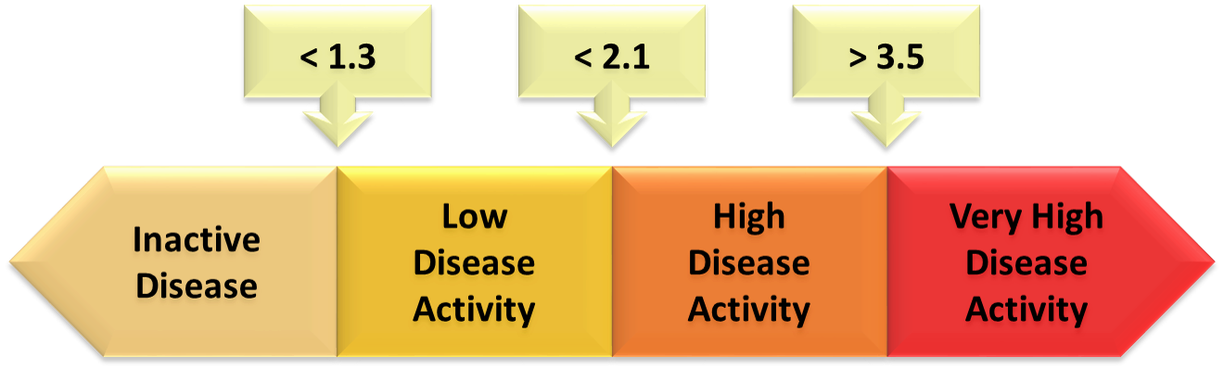
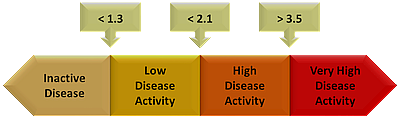
ASDAS calculator - ASAS

Tofacitinib for the treatment of ankylosing spondylitis: a phase III, randomised, double-blind, placebo-controlled study
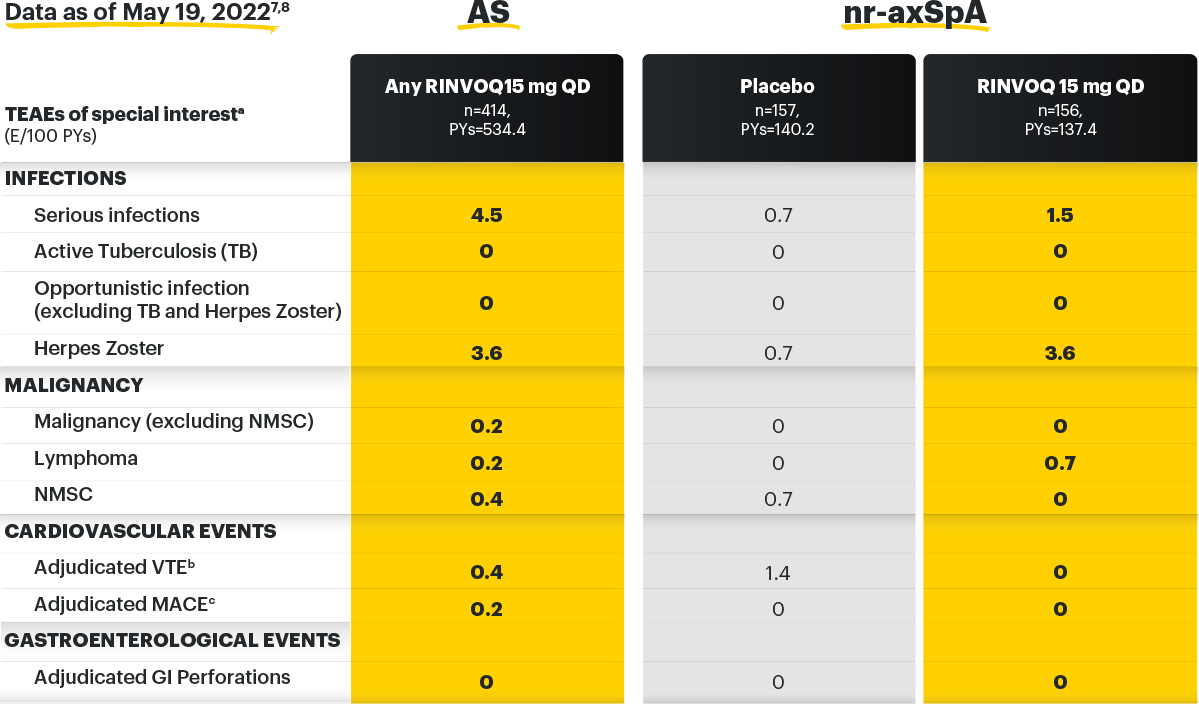
Safety Profile, Axial Spondyloarthritis

Safety and Efficacy of Upadacitinib in Patients With Active Ankylosing Spondylitis and an Inadequate Response to Nonsteroidal Antiinflammatory Drug Therapy: One‐Year Results of a Double‐Blind, Placebo‐Controlled Study and Open‐Label Extension - Deodhar

PDF] ASAS40 and ASDAS clinical responses in the ABILITY-1 clinical trial translate to meaningful improvements in physical function, health-related quality of life and work productivity in patients with non-radiographic axial spondyloarthritis
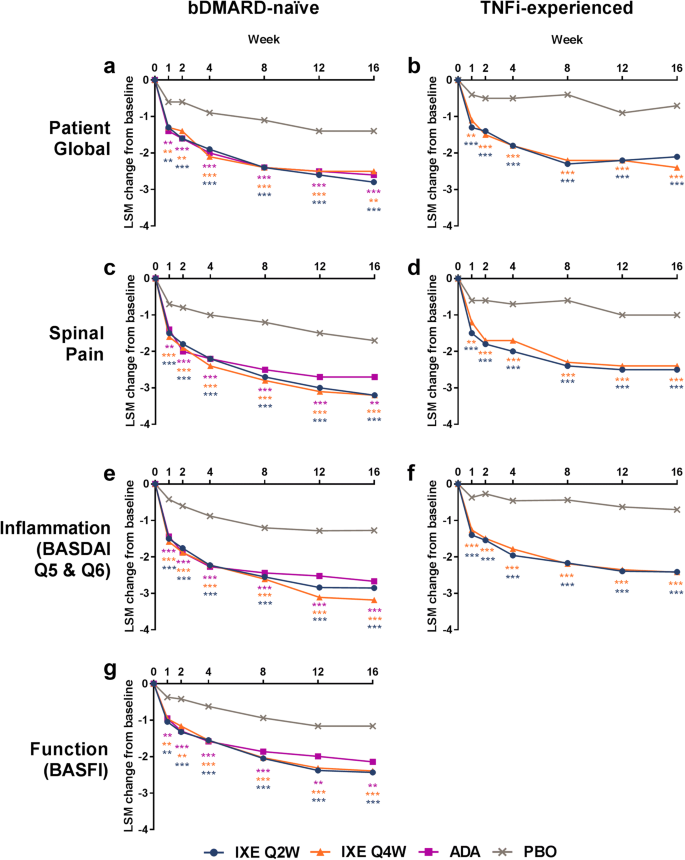
Translating Improvements with Ixekizumab in Clinical Trial Outcomes into Clinical Practice: ASAS40, Pain, Fatigue, and Sleep in Ankylosing Spondylitis
Disease Control Data, Ankylosing Spondylitis

Assessment of SpondyloArthritis international Society criteria for 20%

Table 6, Details of Included Studies - Upadacitinib (Rinvoq) - NCBI Bookshelf
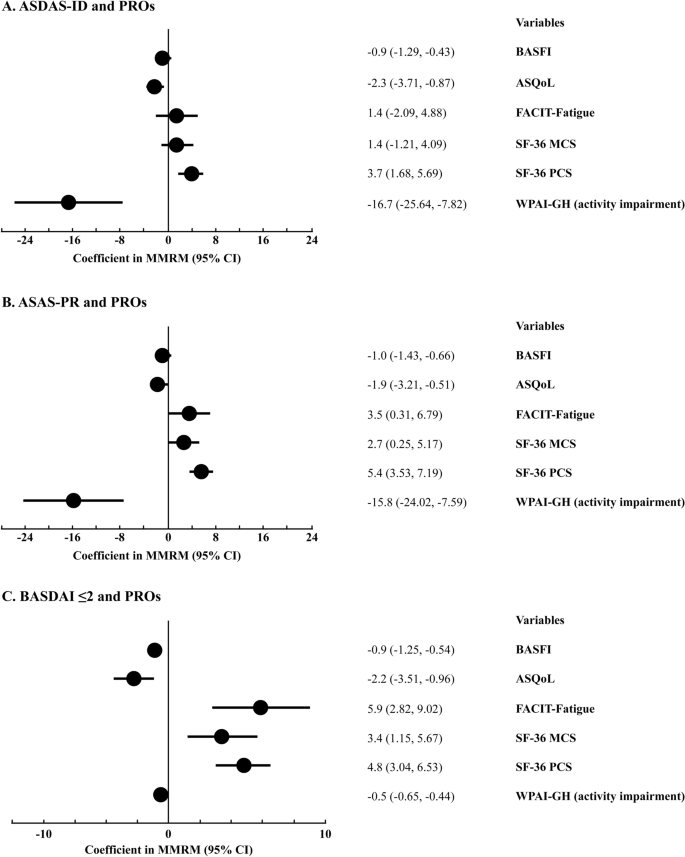
Achievement of Remission Endpoints with Secukinumab Over 3 Years in Active Ankylosing Spondylitis: Pooled Analysis of Two Phase 3 Studies

ARA Abstracts - 2020 - Internal Medicine Journal - Wiley Online Library
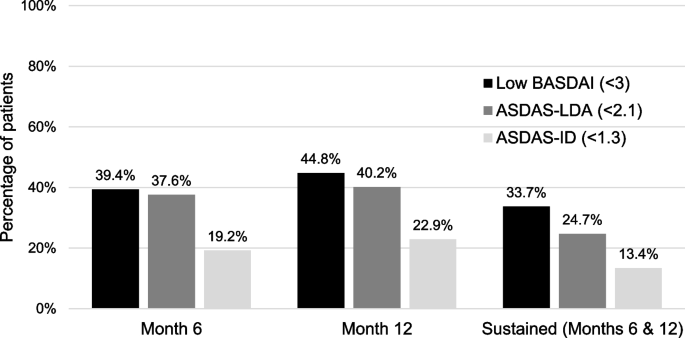
Sustained low functional impairment in axial spondyloarthritis (axSpA): which are the primary outcomes that should be targeted to achieve this?, Arthritis Research & Therapy

Baseline characteristics of ASDAS ID and ASAS PR responders and
de
por adulto (o preço varia de acordo com o tamanho do grupo)