Assessing the value of interim analyses in clinical trials - PMLiVE
Por um escritor misterioso
Descrição
lt;p>Why its important and when its needed</p>

Hidden Consequences of Interim Analyses & Adaptive Trial Options - Quantics Biostatistics
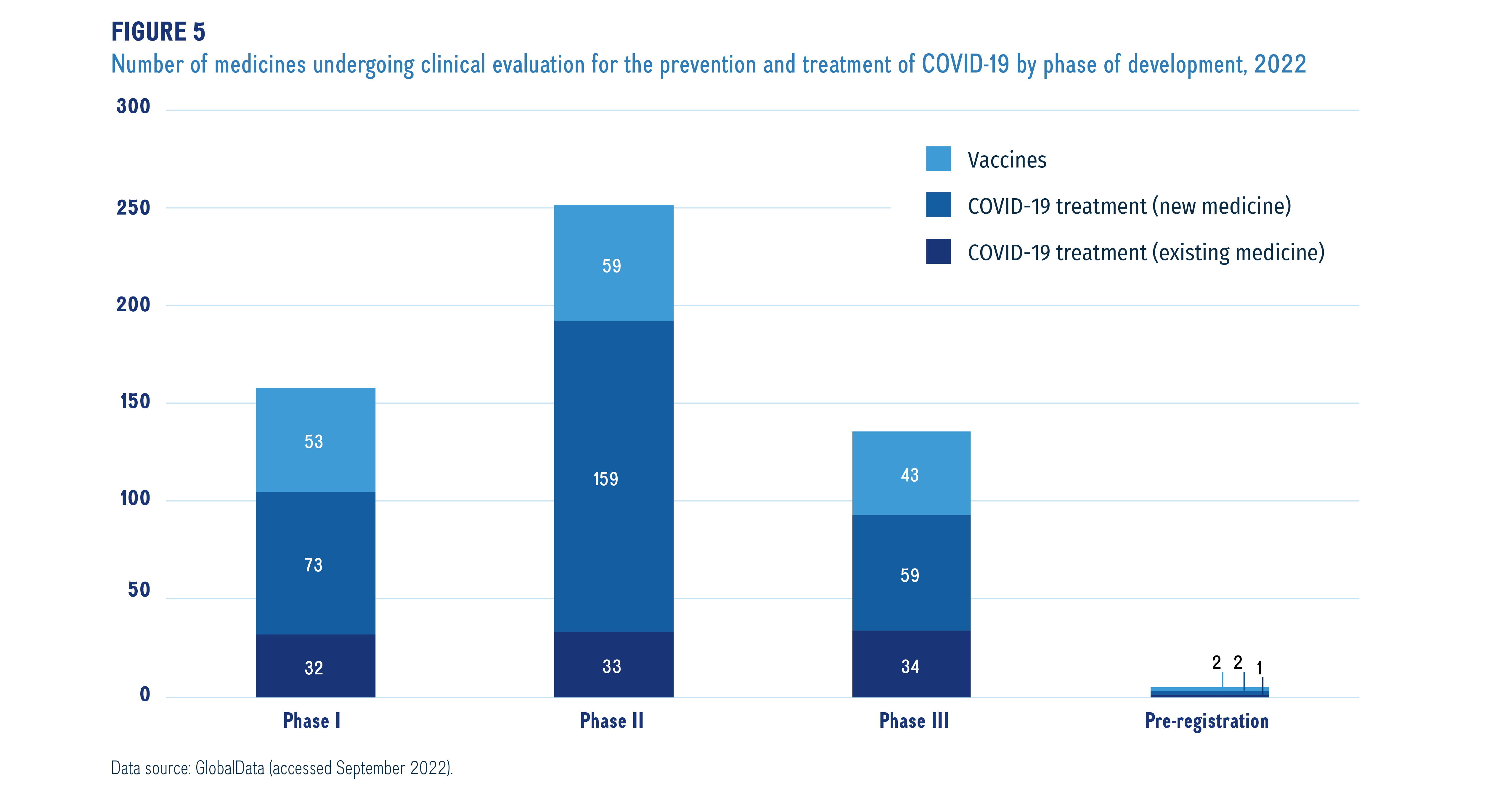
Meds pipeline monitor 2022

Pricing and reimbursement experiences and insights in the European Union and the United States: Lessons learned to approach adaptive payer pathways - Faulkner - 2016 - Clinical Pharmacology & Therapeutics - Wiley Online Library

Calaméo - Pharmafocus July/August 2022

Assessing drugs for ultra-rare conditions in the UK - PMLiVE

AstraZeneca to stop post-marketing study of Andexxa following early success - PMLiVE

To Push or To Pull? In a Post-COVID World, Supporting and Incentivizing Antimicrobial Drug Development Must Become a Governmental Priority
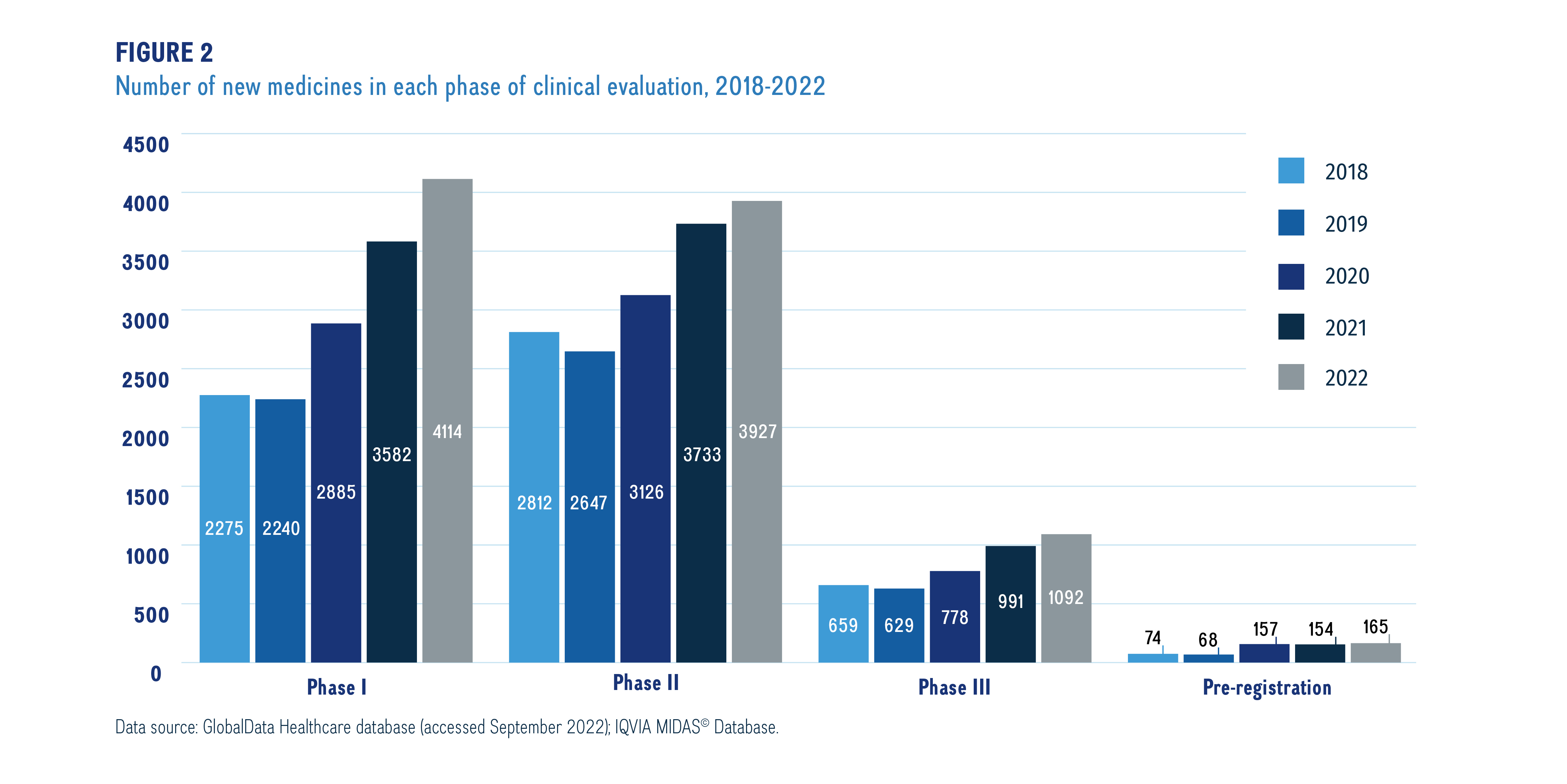
Meds pipeline monitor 2022
image_003.jpg

lantern_logo.jpg

Biogen Scores a Big Win for ADUHELM™, But the Path Ahead Isn't Easy!

Adherence and long-term growth outcomes: results from the easypod™ connect observational study (ECOS) in paediatric patients with growth disorders in: Endocrine Connections Volume 7 Issue 8 (2018)
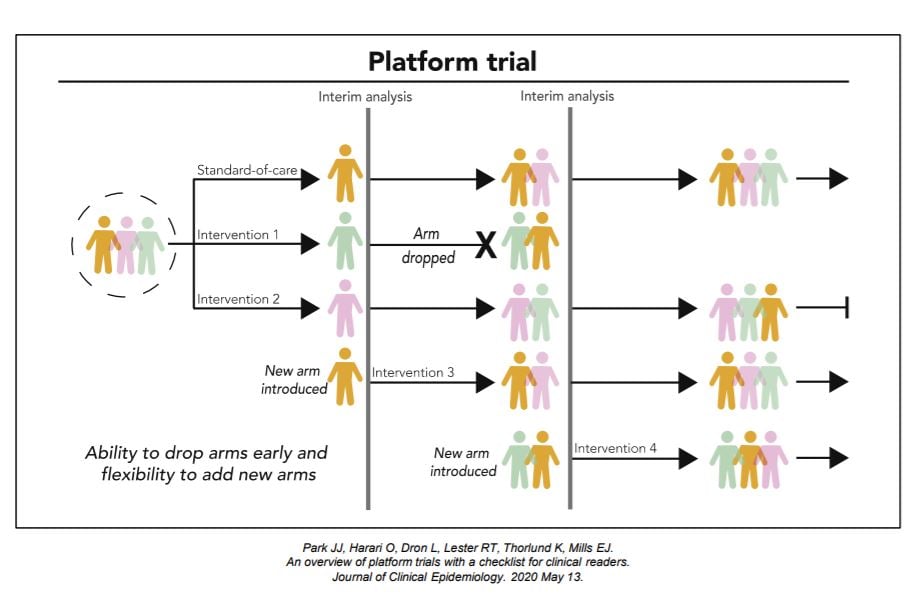
Key Design Considerations for Platform Trials
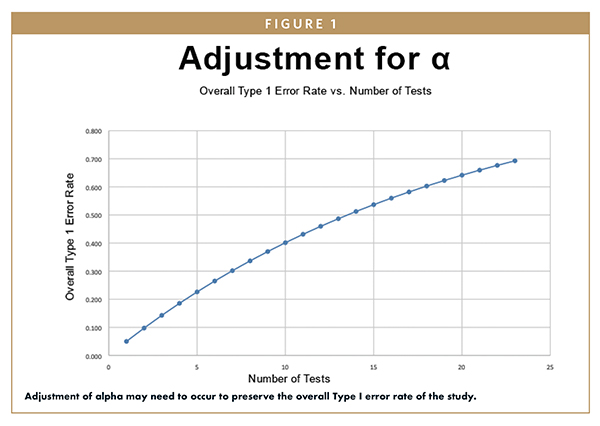
CLINICAL STUDY REPORTING - Assessing the Value of Interim Analyses in Clinical Trials
de
por adulto (o preço varia de acordo com o tamanho do grupo)