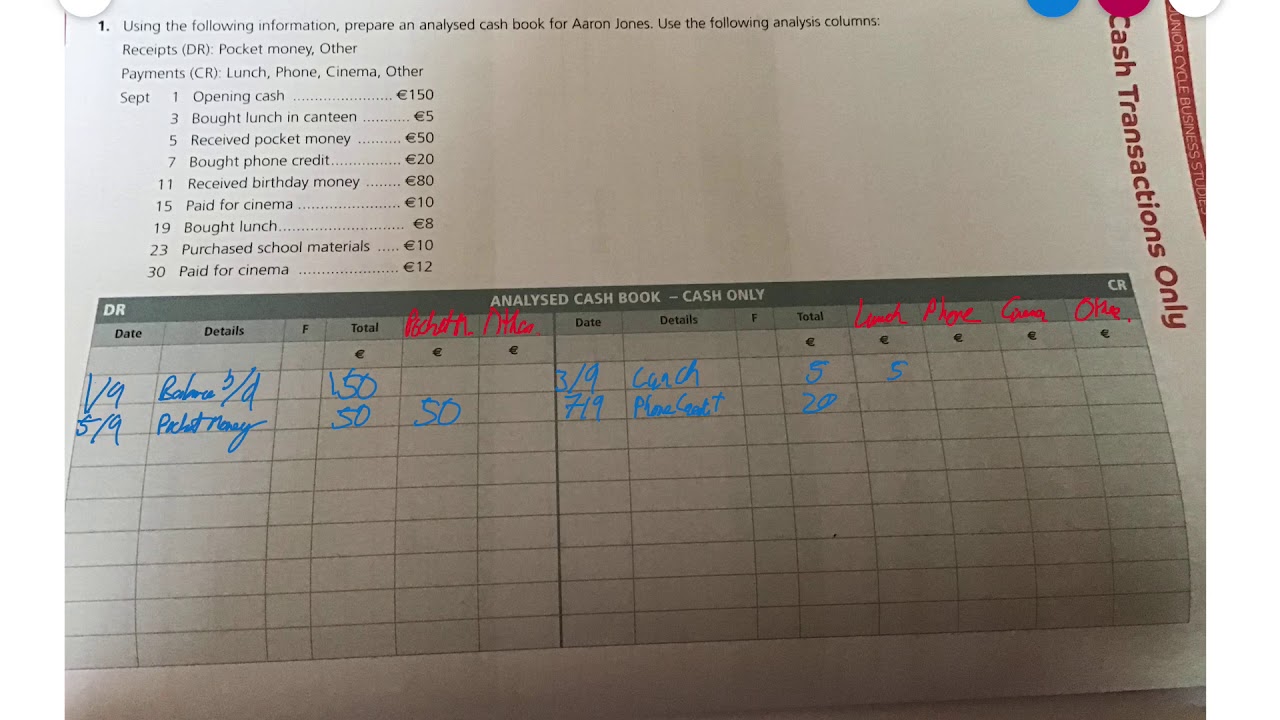
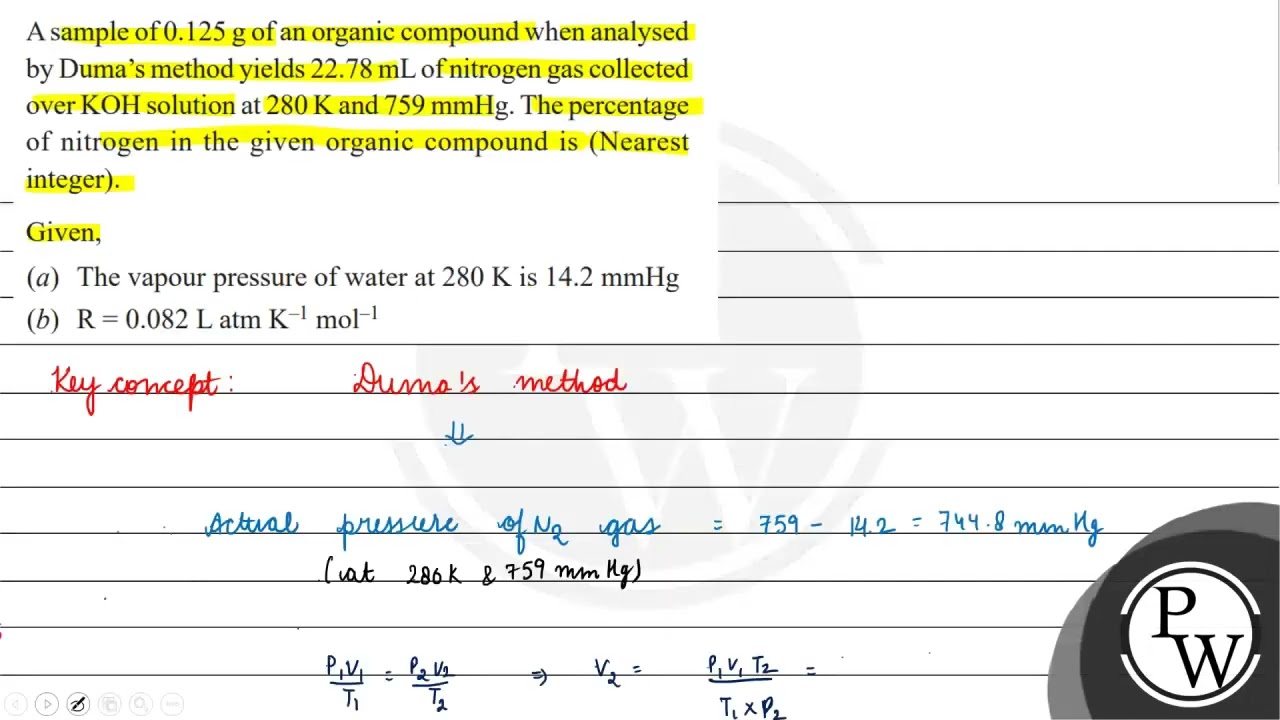
8 (4) A, C 0.1 gm of organic compound was analysed by Kjeldahl's method. In analysis produced NH, absorbed in 30 ml N/5 H,SO. The remaining acid required 20 ml N/10 NaOH
Por um escritor misterioso
Descrição
Click here:point_up_2:to get an answer to your question :writing_hand:84 a c01 gm of organic compound was analysed bykjeldahls method in analysis produced nhabsorbed

0.2 gm of an organic compound was analysed by kjeldahl's method the ammonia evolved was absorbed
21. For the estimation of nitrogen, 1.4 g of an organic compound was digested by Kjeldahl method and the evolved ammonia was absorbed in 60 mL of M/10 sulphuric acid. The unreacted

A sample of `0.50 g` of an organic compound was treated according to Kjeldahl's method.

Estequiometria quimica, Ejercicios de Química

29) 0.2 g of an organic compound was analysed by Kjedahl's method. Ammonia evolved was absorbed an 60 mL N/5 H2SO4. Unused acid required 40 mL of N/10 NaOH complete neutralisation. Find

Study Notes on Analytical Chemistry, EB 3247 - Analytical Chemistry - Nilai

1.216 gm of an organic compound was reacted under Kjeldahl's method and the ammonia evolved was

Analytical Chemistry, PDF, Titration

During nitrogen estimation present in an organic compound by Kje

PDF) Learners Guide to Soil Analysis

Kjeldahl Method Questions - Practice Questions of Kjeldahl Method with Answer & Explanations


REVIEW OF FEATURES OF MERCURY CHEMISTRY OF CHIEF INTEREST TO RADIOCHEMISTS, Radiochemistry of Mercury
de
por adulto (o preço varia de acordo com o tamanho do grupo)